|
Molbank 2006 M515 |
Rhodamine B Pentyl Ester
and its Methyl, Ethyl, Propyl, and Butyl Homologues
Justin A. Ross,*,A
Benjamin P. Ross,B,C Kelly L. Cosgrove,B Halina Rubinsztein-Dunlop,A and Ross P. McGearyB,C
ACentre
for Biophotonics and Laser Science,
BSchool of Molecular and Microbial Sciences;
CSchool of Pharmacy;
The
Tel.: +61 7 3366 9583; Fax: +61 7 3365 1242; E-mail: [email protected]
*Author to whom correspondence should be addressed
Received: 22 June2006 / Accepted: 11 August 2006 / Published: 1 December 2006
Keywords: rhodamine, esterification, acetyl chloride, lipophilicity.
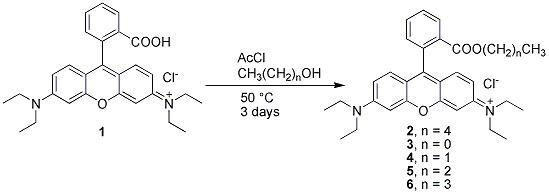
The rhodamines are a highly fluorescent class of compound used in many different fields of research, from the lasing medium in dye lasers to biological stains and markers for cellular drug resistance [1-3]. We are interested in the influence of lipophilicity on the transport of esters of rhodamine in multidrug resistance transporter assays. Herein we describe the synthesis of the novel compound rhodamine B pentyl ester (2), and deposit the spectral data of the known methyl (3), ethyl (4), propyl (5), and butyl (6) homologues [4]. Ester 2 was prepared by a slightly modified facile method [5] that utilizes anhydrous hydrogen chloride generated in situ by the addition of AcCl to a mixture of the rhodamine B free acid (1) and n-pentanol.
Rhodamine B pentyl ester (2)
N-[6-(Diethylamino)-9-[2-(pentyloxycarbonyl)phenyl]-3H-xanthen-3-ylidene]-N-ethylethanaminium chloride
Acetyl chloride (1.25 mL, 17.6 mmol) was added dropwise to a stirred mixture of rhodamine B (1, 100 mg, 0.21 mmol) and n-pentanol (25 mL), and the reaction was heated at 50°C under an atmosphere of argon. After 2 days the reaction was ~ 90% complete by TLC, and additional acetyl chloride (250 µL, 3.52 mmol) was added to drive the reaction to completion. After a further 24 h TLC indicated no remaining rhodamine B (1) and the solution was evaporated in vacuo (water bath temperature < 50 °C to avoid degradation of the product) to afford rhodamine B pentyl ester (2), which was contaminated with a small amount of n-pentanol. The molar ratio of rhodamine B pentyl ester (2):n-pentanol was ~ 3:1 by 1H NMR. To separate the ester (2) from residual alcohol, the crude product was dissolved in water (100 mL) and washed with ethyl acetate (3 × 50 mL). Acetonitrile (35 mL) was added to the aqueous layer and this solution was lyophilised to afford the title compound (2), a hygroscopic amorphous solid (85%).
TLC Rf 0.41 (silica gel 60 F254 aluminum sheets, n-butanol:water:ethanol 9:2:1).
ES-MS, m/z: 513 [M+H]+.
1H NMR (500 MHz, d6-DMSO) d 8.24 (1H, dd, J = 7.9 and 1.2 Hz), 7.91 (1H, dt, J = 7.5 and 1.3 Hz), 7.85 (1H, dt, J = 7.7 and 1.3 Hz), 7.51 (1H, dd, J = 7.5 and 1.1 Hz), 7.11 (2H, dd, J = 9.6 and 2.4 Hz), 7.02-6.99 (4H, m), 3.89 (2H, t, J = 6.2 Hz), 3.65 (8H, br q, J = 7.1 Hz), 1.20 (12H, br t, J = 7.0 Hz) overlapping with ~1.18-1.13 (2H, m), 1.06 (2H, sextet, J = 7.4 Hz), 0.88-0.82 (2H, m), 0.71 (3H, t, J = 7.4 Hz).
13C NMR (100 MHz [6], d6-DMSO) d 165.1, 157.6, 157.1, 155.1, 133.0,
132.7, 131.0, 130.9, 130.5, 130.4, 129.9, 114.6, 112.9, 95.9, 65.2, 45.3, 27.5,
27.4, 21.7, 13.6, 12.4.
UV-visible (H2O): λmax 559 nm.
HRMS calcd for [M+H]+ 513.3117, found 513.3122.
The methyl (3), ethyl (4), propyl (5) and butyl (6) homologues of 2 were synthesized by the same method except that evaporation of the solution in vacuo afforded the ester (3-6) pure, without the need for partitioning between water and ethyl acetate. Previously these compounds were isolated as the bromide, iodide or perchlorate salts, and their spectra were recorded in CDCl3 [4]. Herein we deposit the spectra of the chloride salts (3-6) recorded in d6-DMSO.
Rhodamine B methyl ester (3)
N-[6-(Diethylamino)-9-[2-(methoxycarbonyl)phenyl]-3H-xanthen-3-ylidene]-N-ethylethanaminium chloride
TLC Rf 0.35 (silica gel 60 F254 aluminum sheets, n-butanol:water:ethanol 9:2:1).
ES-MS, m/z: 457 [M+H]+.
1H NMR (500 MHz, d6-DMSO) d 8.27 (1H, dd, J = 7.9 and 1.2 Hz), 7.92 (1H, dt, J = 7.6 and 1.3 Hz), 7.84 (1H, dt, J = 7.7 and 1.3 Hz), 7.51 (1H, dd, J = 7.5 and 1.1 Hz), 7.08 (2H, dd, J = 9.6 and 2.5 Hz), 6.99-6.97 (4H, m), 3.65 (8H, br q, J = 7.02 Hz), 3.60 (3H, s), 1.21 (12H, br t, J = 7.0 Hz).
13C NMR (75 MHz, d6-DMSO) d 165.0, 157.9, 157.1, 155.1, 133.4, 133.3,
130.8, 130.8, 130.5, 130.5, 129.2, 114.6, 112.8, 95.9, 52.5, 45.3, 12.5.
UV-visible (H2O): λmax 558 nm.
HRMS calcd for [M+H]+ 457.2491, found 457.2481.
Rhodamine B ethyl ester (4)
N-[6-(Diethylamino)-9-[2-(ethoxycarbonyl)phenyl]-3H-xanthen-3-ylidene]-N-ethylethanaminium chloride
TLC Rf 0.37 (silica gel 60 F254 aluminum sheets, n-butanol:water:ethanol 9:2:1).
ES-MS, m/z: 471 [M+H]+.
1H NMR (500 MHz, d6-DMSO) d 8.25 (1H, dd, J = 7.9 and 1.1 Hz), 7.91 (1H, dt, J = 7.6 and 1.4 Hz), 7.84 (1H, dt, J = 7.7 and 1.2 Hz), 7.52 (1H, dd, J = 7.6 and 1.1 Hz), 7.09 (2H, dd, J = 9.6 and 2.5 Hz), 7.01-6.99 (4H, m), 3.98 (2H, q, J = 7.1 Hz), 3.65 (8H, br q, J = 7.0 Hz), 1.21 (12H, br t, J = 7.0 Hz), 0.90 (3H, t, J = 7.1 Hz).
13C NMR (75 MHz, d6-DMSO) d 164.7, 157.7, 157.1, 155.1, 133.1, 133.0,
130.9, 130.7, 130.5, 130.4, 129.7, 114.6, 112.9, 95.9, 61.0, 45.4, 13.4, 12.4.
UV-visible (H2O): λmax 559 nm.
HRMS calcd for [M+H]+ 471.2648, found 471.2643.
Rhodamine B propyl ester (5)
N-[6-(Diethylamino)-9-[2-(propoxycarbonyl)phenyl]-3H-xanthen-3-ylidene]-N-ethylethanaminium chloride
TLC Rf 0.39 (silica gel 60 F254 aluminum sheets, n-butanol:water:ethanol 9:2:1).
ES-MS, m/z: 485 [M+H]+.
1H NMR (500 MHz, d6-DMSO) d 8.25 (1H, dd, J = 7.8 and 1.2 Hz), 7.91 (1H, dt, J = 7.6 and 1.3 Hz), 7.85 (1H, dt, J = 7.7 and 1.2 Hz), 7.52 (1H, dd, J = 7.5 and 1.0 Hz), 7.09 (2H, dd, J = 9.6 and 2.5 Hz), 7.02-6.99 (4H, m), 3.89 (2H, t, J = 6.4 Hz), 3.65 (8H, br q, J = 7.0 Hz), 1.29 (2H, sextet, J = 6.9 Hz), 1.20 (12H, br t, J = 7.0 Hz), 0.62 (3H, t, J = 7.4 Hz).
13C NMR (75 MHz, d6-DMSO) d 164.9, 157.7, 157.1, 155.1, 133.1, 132.9,
130.9, 130.8, 130.5, 130.4, 129.8, 114.6, 112.9, 95.9, 66.7, 45.4, 21.1, 12.4,
10.0.
UV-visible (H2O): λmax 559 nm.
HRMS calcd for [M+H]+ 485.2804, found 485.2801.
Rhodamine B butyl ester (6)
N-[6-(Diethylamino)-9-[2-(butoxycarbonyl)phenyl]-3H-xanthen-3-ylidene]-N-ethylethanaminium chloride
TLC Rf 0.40 (silica gel 60 F254 aluminum sheets, n-butanol:water:ethanol 9:2:1).
ES-MS, m/z: 499 [M+H]+.
1H NMR (500 MHz, d6-DMSO) d 8.24 (1H, dd, J = 7.9 and 1.2 Hz), 7.91 (1H, dt, J = 7.5 and 1.4 Hz), 7.84 (1H, dt, J = 7.7 and 1.4 Hz), 7.51 (1H, dd, J = 7.6 and 1.1 Hz), 7.10 (2H, dd, J = 9.6 and 2.5 Hz), 7.02-7.00 (4H, m), 3.91 (2H, t, J = 6.2 Hz), 3.65 (8H, br q, J = 7.2 Hz), 1.21 (12H, br t, J = 7.0 Hz) overlapping with ~1.20-1.15 (2H, m), 0.94 (2H, sextet, J = 7.6 Hz), 0.68 (3H, t, J = 7.4 Hz).
13C NMR (75 MHz, d6-DMSO) d 165.0, 157.5, 157.1, 155.1, 133.0, 132.7,
130.9, 130.7, 130.4, 130.3, 129.8, 114.6, 112.8, 95.8, 64.9, 45.3, 29.8, 18.4,
13.4, 12.4.
UV-visible (H2O): λmax 559 nm.
HRMS calcd for [M+H]+ 499.2961, found 499.2973.
Acknowledgments
We
thank Mr. Graham MacFarlane (
References
1. Zollinger, H. Color Chemistry : Syntheses, Properties, and Applications of Organic Dyes and Pigments, 2nd rev. ed.; VCH: Weinheim, 1991.
2. Gordon, P. F.; Gregory, P. Organic
Chemistry in Colour; Springer-Verlag:
3. Abrahart, E. N.; Dyes
and Their Intermediates, 2nd ed.; Edward Arnold:
4. Ramos, S. S.; Vilhena,
A. F.;
5. Ross, J. A.; Ross, B. P.; Rubinsztein-Dunlop, H.; McGeary, R. P. Synth. Commun. 2006, 36, 1745-1750.
6. The 13C NMR spectra of 3-6 were run at 75 MHz whereas the 13C NMR spectrum of 2 was recorded at 100 MHz to enable resolution of the resonances which appeared at 27.46 and 27.43 ppm in the 100 MHz spectrum.
© 2006 MDPI. All rights reserved.