|
Molbank 2006, M516 |
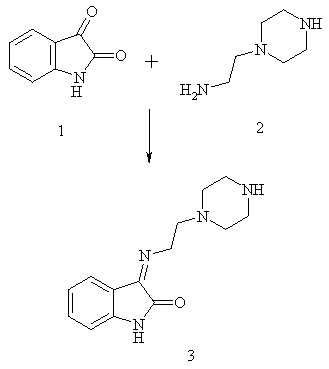
Synthesis of 3-[(Z)-2-piperazin-1-yl-ethylimino]-1,3-dihydro indol-2-one as a novel Schiff base
Ramakrishna Reddy.K
and Mahendra.K.N*
Department of Chemistry,
E-mail: [email protected], [email protected]
*Author to whom correspondence should be addressed
Received: 9 September 2006 / Accepted: 20 October
2006 / Published: 1 December 2006
Keywords: isatin, Schiff base, 1-(2-aminoethyl) piperazine
Isatin (1H-indole-2,3-dione) was first obtained by Erdman and Laurent in
1841 as a product from the oxidation of indigo by nitric and chromic acids [1].
The synthetic versatility of isatin has led to the
extensive use of this compound in organic synthesis. In nature, isatin is found in plants of the genus Isatis [2], in Calanthe
discolor LINDL [3]. Isatin is the biologically active chemical produced by
an Altermones sp. Strain inhabiting the
surface of embryos of the cardiean shrimp Palaemon macrodectylus,
which protect them from the pathogenic fungus Lagenidium callinectes
[4]. Schiff bases of isatin were reported to possess
interesting biological activities such as anti-HIV [5-7], anticonvulsant
[8], antibacterial [9-11], antiprotozoal
[12, 13], antifungal [14-16], antiviral [17-19], anthelminthic [20,21], antidepressant [22] anti-inflammatory and antitumour [23] activities. The Schiff bases of isatin have also been used as a ligand
for complexation with various metal ions [24].In view
of the potent biological activities, we decided to synthesize a new isatin Schiff base by the
condensation of isatin with 1-(2-aminoethyl)piperazine (AEP). Its biological activities and chelating behaviour
with various metal ions is under study.
Ethanolic solutions of isatin (1) (0.01mol, 1.47g) in 50 ml and 1-(2-aminoethyl) piperazine (2) (0.01mol, 1.8ml, 1.79g) in 50 ml were mixed and refluxed for about 2 hours. The reaction mixture was evaporated to a small volume and left to cool. The resulting Schiff base (3) ligand precipitated on cooling and then was filtered off, washed with ethanol and recrystallised from ethanol. The purity of the Schiff base ligand was monitored on TLC using eluants 1:1 ethyl acetate and petroleum ether and separated by column chromatography (Yield =90%).
Melting point: 220กใC
Elemental analysis: Calculated for C14H18N4O C, 60.86; H, 7.24; N, 20.29. Found: C, 60.28; H, 7.20; N, 19.97.
IR (KBr, cm-1):
3239.9 (N-H), 1714.6 (C=O), 1618.6 (C=N)
MS (ESI, m/z): 259 [M+]
1H-NMR (400MHz, DMSO-d6): 10.3(1H, s), 7.6-6.8 (4H, m), 2.8-2.1(13H, m).
13 C-NMR (400MHz, DMSO-d6): 38.3; 45.6; 54.3; 61.8; 110.6; 121.9;
122.6; 123.3; 131.6; 134.3; 141.6; 162.0.
References
1. Dasilva, J. F. M.; Garden, S. J.; Pinto,
A. C. J. Braz. Chem. Soc. 2001,
12(3), 273.
2. Guo, Y.; Chen, F.; Zhongcaoyao 1986, 17, 8. (CA 104:213068f).
3. Yoshikawa, M.; Murakami, T.; Kishi, A.; Sakurama, T.; Matsuda, H.; Nomura, M.; Matsuda, H.; Kubo, M. Chem. Pharm. Bull. 1998, 46, 886.
4. Gil-Turners, M. S.; Hay, M. E.; Fenical, W. Science, 1989, 116.
5. Pandeya, S. N.; Yogeeswari, P.; Sriram, D.; De Clercq, E.; Pannecouque, C.; Witvrouw, M. Chemotherapy, 1999, 45, 192-196.
6. Pandeya, S. N.; Sriram, D.; Nath, G.; De Clercq, E. Eur. J. Med. Chem. 2000, 35, 249-255. Pandeya,
S. N, Sriram, D.; Nath, G.;
De Clercq, E. Arzneimittel Forschun./Drug
Res.2000,50, 55-59.
8. Sridhar, S. K.; Pandeya, S. N.; Stables, J. P.;
Armes, S. K. Eur. J. Pharm. Sci. 2002, 16, 129.
9. Pandeya, S. N.; Sriram,
D. Acta Pharm. Turc. 1998, 40, 33.
10. Sarangapani, M.; Reddy, V. M. Indian J. Pharm.
Sci. 1994,
56, 174.
11. Varma, R. S.; Nobles, W. L. J. Pharm.
Sci. 1975, 64, 881.
12. Imam, S. A.; Varma, R. S. Experientia, 1975, 31, 1287.
13. Varma, R. S.; Khan,
14. Pandeya, S. N.; Sriram, D.; Nath, G.; De Clercq, E. Indian J.
Pharm. Sci.1999,61, 358.
15. Pandeya, S. N.; Sriram, D.; Nath, G.; De Clercq, E. Sci.Pharm. 1999,
67,10.
16. Pandeya, S. N.; Sriram, D.; Nath, G.; De Clercq, E. Pharm. Acta Helv.
1999,74,11.
17. Varma, R. S.;
Nobles, W. L. J. Med. Chem. 1967, 10, 972.
18.
Singh, S. P.; Shukla, S. K.; Awasthi,
L. P. Curr. Sci.
1983,52,766.
19. Logan, J. C.; Fox, M. P.;
Morgan, J. M.; Makohon, A. M.; Pfau, C. J. J. Gen. Virol. 1975, 28, 271.
20. Sarciron, S. E.; Audin, P.; Delebre, I.; Gabrion, C.; Petavy, A. F.; Paris, J. J. Pharm.
Sci. 1993, 82, 605.
21. Et-savi, E. A.; Mostafa, T. B.; Mostafa, B. B. J. Egypt. Soc. Parasitol.
1998, 28, 481.
22. Seshaiah.K.S and Atmakuru.R,
Biol.Pharm.Bull.2001,24,1149.
23. Mohanan.K and Murukan.B, Syn. and. rea. in Inorg. Met-org. and Nano-Met. Chem. 2005,35,837.
24. Rama Krishna Reddy.K.;Madhusudan Reddy.K and Mahendra.K.N, Ind.J.of
Chem,2006,45A,377.
© 2006 MDPI. All rights reserved.