|
Molbank 2006, M518 |
Synthesis of
2-(2-napthylthio)-quinoline-3-carboxaldehyde as a novel complexing
agent
Bennehalli Basavaraju a*, Halehatti S. Bhojyanaikb and Mustur C. Prabhakarab
aDepartment of Biotechnology, GM Institute of
Technology, Davangere-577 006,
bDepartment of Industrial Chemistry, School
of Chemical Sciences, Kuvempu University,
Shankaraghatta-577 451, Shimoga, Karnataka, India
Phone:
+91 8192 233377, Fax: +91 8192 233344, e-mail: [email protected]
* Author
to whom correspondence should be addressed
Received:
Keywords: 2-Chloroquinoline-3-carboxaldehyde, 2-(2-napthylthio)-quinoline-3-carboxaldehyde, 2-naphthalenethiol.
Abstract:
In this paper
we propose the synthesis of 2-(2-napthylthio)-quinoline-3-carboxaldehyde. In addition to its synthesis we present elemental,
IR, and NMR spectral analysis to characterize the molecule.
Introduction
In the last decade, much attention has been given to the organic ligands and transition metal complexes because of their biological relevance, interesting spectral and magnetic properties. The fused aromatic heterocyclic ligands and their metal complexes are being used extensively as pharmaceutical and chemotherapeutic agents [1-4]. On the other hand, quinoline and their derivatives form an interesting class of compounds which display attractive applications as pharmaceuticals [5-8] and are general synthetic building blocks, due to their chemical and biological relevance. Therefore, it was thought worthwhile to isolate and characterize some novel quinoline derivatives containing different donor atoms.
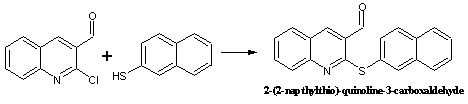
Synthesis of
2-(2-napthylthio)-quinoline-3-carboxaldehyde
Into a mixture of 2-Chloroquinoline-3-carboxaldehyde (0.958 g, 5 mmol), 2-naphthalenethiol (0.8 g, 5 mmol) and potassium carbonate (1.38 g, 10 mmol), anhydrous dimethylformamide (50 ml) was added. The mixture was heated at 80-90 oC for 2 h with constant stirring and was then cooled to room temperature. The product was separated on an alumina column (3x20 cm) using methylene chloride/acetonitrile (5:1) as eluant.
Melting point: 156-160 oC
Elemental Analysis: Calculated for C20H13NOS (315.38): C 76.16, H 4.15, N 4.44; found C 76.35, H 4.32, N 4.63.
IR (KBr, cm-1): 760 (-CSC-), 1710 (aldehyde, CHO), 1570 (C=C), 1650 (C=N), 2950 (C-H, aromatic).
1H-NMR (250 MHz, DMSO-d6): ¦Ä= 9.6 (s, 1H, -CHO), 7.4-8.6 (m, 11H, Ar-H).
13C-NMR (62.9 MHz,CDCl3): 128.9; 127; 130; 131; 149; 130; 141; 159; 147; 170; 125; 123; 125; 135; 126; 134; 129; 127; 128; 129.
Acknowledgment
The authors gratefully acknowledge
financial support from Mr. G.M. Lingaraju, Secretary,
GM Institute of Technology, Davangere-577 006,
References
1. P.M.O¡¯Neill, P.G.Bray, S.R.Hawley, S.A.Ward and B.K.Park, Pharmacol.Ther., 1998, 77(1), 29.
2. M.Foley and L.Tilley, Pharmacol.Ther., 1998, 79(1), 55.
3. D.J.Krogstad, I.Y.Gluzman, D.E.Kyle, A.M.Oduola, S.K.Martin, W.K.Millhouos and P.H.Schlesinger, Science, 1987, 238, 1283.
4. T.E.Wellems, A.Walker-Jonah and L.J.Panton, Proc.Natl.Acad.Sci.U.S.A, 1991, 88(8), 3382.
5. P.B.Madrid, N.T.Wilson, J.L.DeRisi, and K.Guy, J.Comb.Chem., 2004, 6, 437.
6. E.Lukevics, I.Segal, A.Zablotskaya and S.Germane, Molecules, 1997, 2, 180.
7. V.I.Kozlovski, V.P.Vdovichenko, S.Chlopicki, S.S.Malchik, K.D.Praliyev and O.T.Zcikibayev, Pol. J.Pharmacol., 2004, 56, 767.
8. N.Noda, Y.Yashiki, T.Nakatani, K.Miyahara and X.M.Du, Chem.Pharm.Bull., 2001, 49(7), 930.
© 2006 MDPI. All rights reserved.