|
Molbank
2006, M519 |
Synthesis and Protonation Constants of an Amide-Based Chelating Cyclophane
Julio
Cesar Altamirano-Coronadoa, Carolina
Godoy-Alcántarb, Felipe Medranoa*
aDepartamento de
Investigación en Polímeros y Materiales. Universidad de Sonora. Apartado
Postal 130, Col Centro.83000. Hermosillo, Sonora, México. Tel +52+662+2592161, Fax +52+662+592216.
email: [email protected], [email protected]
bCentro de Investigaciones Químicas, Universidad Autónoma del
Estado de Morelos. 62209.
*Author to whom
correspondence should be addressed
Received:
Keywords: Cyclophane, potentiometry,
protonation, amine, anhydride, condensation.
Introduction
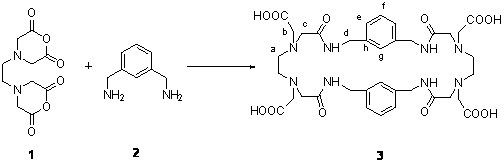
Our research group have reported that, under high dilution conditions,
condensation reactions between 4,4’-ethylenebis(2,6-morpholinedione), 1,
and aromatic diamines gave a new series of tetraaza chelating cylophanes,
which have amide and aromatic groups in the ring framework and pendant carboxymethyl groups [1]. The resulting functionalized macrocycles have novel coordination and structural properties
due to the unique arrangement of different types of donor groups [2]. In this
work, we have employed the aromatic diamine 1,3-bis(aminomethyl)benzene, 2, and obtained a new chelating
cyclophane (3),
4,12,17,24-tetraoxo-6,9,19,22-tetrakis(carboxymethyl)-3,6,9,12,16,19,22,25-octaaza-1,4(1,3)-dibenzacyclohexacosano .
Synthesis
of 3
Cyclophane 3 was obtained by the
condensation reaction between 1 and 2 under high dilution
conditions as was reported for other similar cyclophanes
[1]. A dimethylformamide (DMF,
Aldrich) solution (50 mL) containing 1 g of 1,3-bis(aminomethyl)benzene
(Aldrich) was slowly added to 2.32 g of 4,4’-ethylenebis(2,6-morpholinedione)
(Aldrich) in 250 mL of DMF
with vigorous stirring during 2 hours. After the resulting reaction mixture was
left to stand over night, any solid formed was removed by filtration and the
filtrate was concentrated to a viscous liquid (ca. 10 mL). Addition of ethanol (20 mL) to the liquid gave a pale yellow solid, which was
separated by filtration. The crude solid was dissolved in 30 mL of boiling water. Cooling the resulting solution gave a
colorless solid. The product was recovered by filtration, washed with water and
dried under vacuum. Yield 15.3 %.
Spectroscopic
Measurements
The solution
electronic spectra were obtained by the use of a Perkin-Elmer Lambda 2 UV-vis spectrometer. The
emission spectra were recorded on a Perkin Elmer LS-50 spectrofluorometer.
The pH of the sample solutions was adjusted by adding a minimum amount of
dilute NaOH solution or solid Na2CO3.
The electrospray ionization (ESI) mass spectra were
obtained by the use of a JEOL HX 110A spectrometer for sample solutions of an
ammonia-methanol (5:95)
mixture. The NMR spectra were obtained on a Bruker Avance 400 or Varian Unity 200 spectrometer in D2O
with reference to sodium 2,2-dimethyl-2-silapentane-5-sulfonate,
DSS. The infrared spectra were recorded on a Perkin-Elmer 1600 FT IR
spectrophotometer and samples were analyzed as KBr
pellets. The
Elemental Analysis was performed by Centro de Investigaciones Químicas,
Universidad Autónoma del Estado de Morelos. Cuernavaca, Mexico.
Anal. Calc. for C36H48N8O12(H2O)3:
C,51.52; H,6.49; N, 13.36%; found: C,51.67; H,6.48; N, 13.47% .
1H NMR (D2O-Na2CO3, pD 10.3, 400 MHz, reference
DSS): d = 7.29 (t, 2H, Hf), 7.15 (s, 2H, Hg), 7.13 (d, 4H, He),
4.24 (s, 8H, Hd), 3.17 (s, 8H, Hc), 3.09 (s, 8H, Hb),2.60
(s, 8H, Ha).
13C NMR (D2O-Na2CO3, pD 10.3, 50 MHz,
reference DSS): d = 179.17 (-COO-), 174.14
(-CO-NH),138.30 (Ch), 129.32 (Cg), 128.90 (Cf),
126.76 (Ce), 59.18 (Cc), 58.43
(Cb), 52.59 (Ca),42.85 (Cd).
IR: 3335 cm-1 (N-H, amide), 1728 cm-1
(C=O, Carboxylate), 1668 cm-1 (-CO-NH-, Amide I, ), 1641 cm-1
(-CO-NH-, Amide II), 798 cm-1 (aromatic), 709 cm-1
(aromatic).
UV
(aqueous solution, pH 9.0 I = 0.1 M KCl): 260 nm (e = 510 M-1 cm-1).
Fluorescence
(aqueous solution, pH 9.0, I = 0.1 M KCl): Emission
band at 290 nm (lexcitation = 260 nm).
ESI MS: The electrospray
mass spectrum of 3 in an ammoniacal methanol
solution (Figure 1) exhibited, in addition to the [M +H]+
peak (m/z = 785.3, 100%), an extra peak at m/z =
393.2 (12%) [M+2H]2+.The
intervals between the isotope peaks proved that the latter species also had z
= 2.
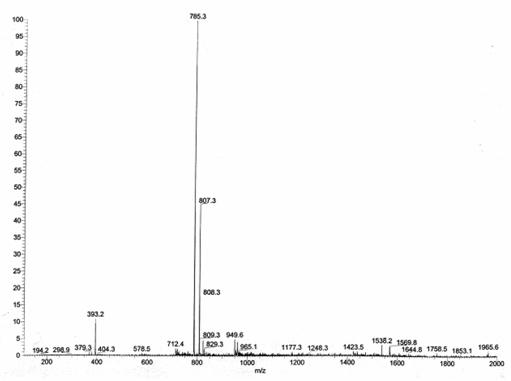
Figure
1. ESI
mass spectrum of 3.
Protonation Constants
Coordination [2], molecular
recognition [3], spectroscopic[1] and structural [4]
properties of this class of compounds are strongly pH dependent, therefore the
precise determination of their acidity constants is very important. The protonation constants of the cyclophane
were determinated by potentiometry.
The titrations were carried out at 298.1 ± 0.1 K using KCl 0.1 M as supporting electrolyte in a sealed-jacketed
vessel under nitrogen with a piston type burette and a Thermo Orion model
920Aplus pHmeter equipped with an Orion 8102U
combination electrode. The glass electrode was calibrated as a hydrogen-ion
concentration probe by titration of previously standardized amounts of HClO4
with CO2-free NaOH solutions and determinated the equivalent point by Gran’s
method [5], which gives the ionic product of water (pKw
= -13.95). The computer program HYPERQUAD 2000 [6] was used to calculate the protonation constants. The pH range investigated was 3
– 11 and the concentration of macrocycle was 1
x 10 –3 M.
Only 4 protonation
constants, of the eight expected, were detected as log K1 = 7.70
(standard deviation = 0.06), log
![]()
![]()
Distribution diagram of the
various species present in solution was calculated using the program SPECIES
(Academic Software) and is shown in Figure 2.
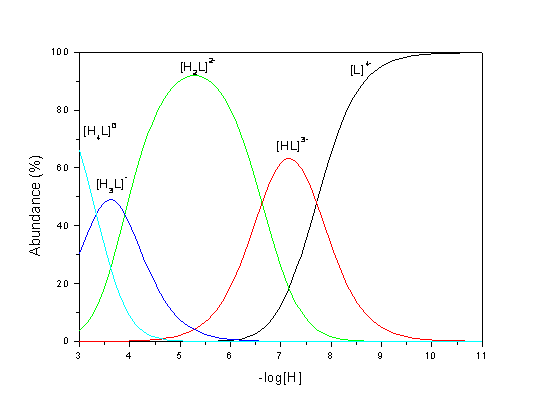
Figure 2. Calculated species
distribution plots for 3.
Based on the reported data for
other similar cyclophanes [1,2]
is possible to assign the observed constants: first and second protonation events occur at amine nitrogen atoms, while
third and fourth protonations occur at carboxylate oxygen atoms.
Acknowledgments
The authors thank CONACyT for the financial
support (grant 39574Q)
References
1. Inoue, M. B; Medrano, F; Inoue, M,
and Fernando,Q. J. Chem. Soc. Perkin Trans. 2,
1998, 2275-2279.
2. Inoue, M. B; Medrano, F; Inoue, M; Raitsimiring, A. and Fernando, Q. Inorg.Chem.,
1997,36, 2335-2340.
3. Virues, C.; Velazquez, E. F.; Inoue, M. B. and M. Inoue, J.
Inclusion Phenom. and Macrocyclic Chem., 2004, 48, 141-146.
4. Altamirano-Coronado, J.C;
Hopfl, H; Godoy-Alcantar, C; Machi, L and Medrano. F. Analytical Sciences:
X-ray structure on line. 2006. in press.
5. Martell, A.E. and Motekaitis. Determination
and use of stability constants. Wiley-VCH: New York, 1992; pp 37-40.
6. Gans, P; Sabatini, A.
and Vacca, A. Talanta,
1996, 43, 1739-1753.
2006 MDPI. All rights reserved.