http://www.chemistrymag.org/cji/2000/023017pe.htm |
|
Zhang Yuhong , Wu Ming , Xiong Gouxing , Yang Weishen
(State Key Laboratory of Catalysts, Dalian Institute of Chemical Physics,The Chinese Academy of Sciences, Dalian 116023, China)
Received
Nov. 24, 1999; Supported by the National Natural Science Foundation of China (Grant No. 29873050) and the Chinese Academy of Sciences (Grant No. KJ951-A1-505).Abstract A new method for the preparation of TiO2 sol by directly peptizing commercial powder has been reported in this paper. It was found that the particle size which was defined by atomic force microscopy (AFM) could be controlled effectively by acidity of the peptized solution. Their absorption spectra showed a typical Q-size effect and two pronounced exciton peaks at 260nm and 310nm, respectively. A strong fluorescence peak at 340nm could be assigned to the transition of the surface Ti-OH groups, suggesting the existence of abundant surface hydroxy groups on the surface of the small TiO2 particles. The relationship between the bandgap and particle size was also discussed through several calibration curves obtained from quantum mechanical calculations.
Keywords sol-gel, titanium dioxide, quantum-size, UV-absorption, fluorescence
1.INTRODUCTION
TiO2 is a semiconductor that has been often investigated in
photoelectro-chemistry and photocatalysis [1]. The understanding of its
photophysical and photochemical properties in aqueous media is of particularly interest
because of its extensive application in the detoxification of polluted ecosystems [2].
It has been known that the electronic band structure of a semiconductor
oxide is size dependent when its dimension is comparable with the exciton (Bohr) radius
(the so-called Q-size effect). The development of the preparation and stabilization method
for monodispersed semiconductor nanoparticles in transparent colloids is very important as
offering a nice opportunity of experimental verification for theoretical predictions.
Systematic studies of the size-dependence of the photo-properties of TiO2 sol,
however, have not kept pace with the studies in heterogeneous photocatalysis where TiO2
play a significant role. The preparation of quantum-size (< 3nm) TiO2
colloidal particles was reported by Kormann et al [3] where a blue shift of the
UV absorption edge was observed with the decrease of the particle size. Anpo et al [4]
had reported the preparation and thereafter the properties of TiO2 powders with
controlled particle size in the range of 5.5-200nm (rutile) and 3.8-53nm (anatase). They
have found distinct Q-size effects even for relatively large crystallites, for instance,
the band gap increased by 0.07 eV relative to the bulk bandgap (3.0 eV) for 12-nm-sized
rutile particles and about 0.16eV relative to the bulk value of 3.2eV for 3.8-nm-sized
anatese particle.
In this article we describe a method for the preparation of colloidal
TiO2 solution with different particle size from a commercial powder. Their
Q-size effects were shown by UV-vis absorption and fluorescence spectra. The relationship
between the particle size and the wavelength of the absorption threshold was discussed in
more detail by several calibration curves obtained from quantum mechanical calculations.
2.EXPERIMENTAL
Commercial powder TiO2 assigned as T-25 in this paper (made by Taixing,
China) in deioned water was hydrated for 1 hour, then was peptized with HCl solution
followed by refluxing for 48 hours under vigorous stirring at 30oC. After
filtering off the unpeptized powder, an aqueous colloidal solution was obtained. This
colloidal solution was stable for weeks at room temperature.
Particle sizes were determined by atomic force microscopy (AFM). UV-vis
absorption spectra were recorded on HITACHI 200-10 spectrophotometer equipped with an
integrating sphere accessory for diffuse reflectance and transmission measurement.
Fluorescence spectra were measured by exciting samples with a 280nm light beam on a
HITACHI MPF-4 fluorescence spectrophotometer.
3. RESULTS AND DISCUSSION
3.1 Preparation of colloidal TiO2
T-25 powder is commercial TiO2, which contains mainly anatase
structure and only a few rutile as defined by XRD analysis. The UV-vis absorption and
fluorescence spectra of T-25 powder were shown in Figure 1. A broad absorption continuum
appears at the range 420nm to 200nm. The onset of absorption is about 410nm, approaching
the energy gap of bulk rutile TiO2 (3.0eV, 410nm). This probably results from a
few rutile in T-25 powder. The fluorescence spectrum shows a broad continuum at 400nm,
which belongs to the fluorescence of Ti-O bond [5], and another weak peak at
340nm. Spectral assignments will be discussed in the following section. All the above
results suggest that the physical and chemical properties of T-25 powder are similar to
those of bulk TiO2 (anatase: Eg=3.2ev,lonset=388nm; rutile: Eg=3.0ev, lonset=410nm).
| Fig.1 The absorption and fluorescence spectra of T-25 powder |
During the preparation
process, the size and the stability of colloidal particles are determined by several
factors such as the acid type, acidity (H+/Ti mole ratio) and temperature. Four
samples named T-1, T-2, T-3 and T-4 were prepared when H+/Ti mole ratio was
1.2, 1.0, 0.7 and 0.4, respectively. The particle size could be effectively controlled by
acidity in the peptized solution [6]. The particle size of TiO2 sol
decreases with the increase of H+/Ti ratio. Moreover, the transparency of these
samples T-1 to T-4 became worse and worse, providing further evidence that the particle
size decreases with the increase of H+/Ti ratio.
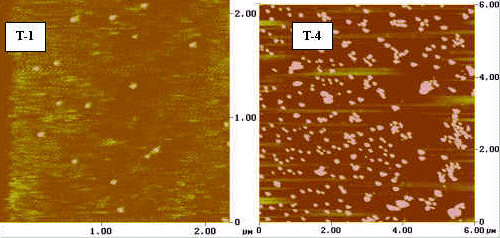
In order to prove this point, we measured the exact particle size of
T-1 and T-4 samples by AFM (see to Fig.2). Fig.2a is the AFM pictures of sol particles.
Fig.2b shows the size distribution of T-1 and T-4. The mainly particle size of T-1 is
1.2nm while that of T-4 is 10.8nm. Their size distributions are both uniform, but the
distribution range of T-4 is broader than that of T-1. The particle size of T-1 is in the
range of 0.2-2.3nm while T-4 has a particle size in the range of 5-30nm. We thus concluded
that the acidity (H+/Ti mole ratio) should be one important factor in
controlling the size of sol particles.
|
|
| Fig.2 (a) AFM characterization and (b) height histogram of sample T-1 and T-4 | |
3.2 UV-vis absorption spectra
Figure 3a shows the UV-vis absorption spectra of TiO2 colloids obtained with
different particle size. The absorption of TiO2 sol has significant difference
from that of T-25 powder. The onset of absorption was obtained by extrapolating the steep
part of the rising absorption curve. The onsets of absorption of T-1, T-2, T-3 and T-4
appear at lonset=342nm, 348nm, 359nm and 369nm, respectively, which exhibit large
blue shifts relative to the onset of bulkanatase (lonset=388nm). Moreover, the onset (lonset) is more and more
rapidly shifted toward shorter wavelength with the increase of the H+/Ti mole
ratio. It is interesting that two pronounced peaks appear at 260nm and 310nm,
respectively, and also their sites keep uncharged with different samples. However, the
relative contents of the two peaks vary with the samples (see to the fitted curves in Fig.
3a). When the particle size is smaller, the relative content of peak at shorter wavelength
260nm becomes higher.
The absorption onsets of the four different colloids are shifted by 0.4~0.2eV with respect
to the absorption onset of the TiO2 anatase crystalline phase. In principal,
this could be due to the small quantum size of our colloidal particles whose radii are
comparable to the exciton Bohr radius. It is well known that the concentration of colloids
and the variety of solvent affect the onset of absorption. Kormann [3] found
that neither variation of the concentration nor dialyzing of TiO2 colloid
solution has any effect on the curve of lnα versus the photon energy, E. Figure 3b shows the plot of lnα versus the photon
energy, E=hcl-1,
for the four samples. The absorption coefficient α has been
calculated from the measured absorbance (A) via
![]()
where p stands for the density of TiO2, 3.9 g.cm-3;
M, the molecular weight, 79.9 g.mol-1; c, the molar concentration of TiO2
and l is the optical path length. The linear partition of curves of colloids was found
between ln α =8 and ln α =13. Urbach’s rule suggests to use the values between ln α
=6 and 10 for the determination of semiconductor bandgaps. Taking these curves, the linear
partition of T-1 and T-2 is between ln α =8.7 and ln
α =9.4 (the circles in Fig. 3b), and the bandgaps of T-1 and T-2 are calculated in
3.4eV and 3.3eV, respectively. However, for T-3 and T-4 samples, the onsets of linear
partition are not obvious and a continual curve appears between ln α
=6 and ln =10, which suggest that the bandgaps of T-3 and T-4 are about 3.0eV.
Therefore, we can conclude that a TiO2 colloid prepared by H+/Ti=1.2
has particle sizes small enough to result in a bandgap shift of 0.2ev without solvent
effect.
|
Fig.3 (a) Absorption spectra
(solid lines) and fitted-cruves (dashed lines) of TiO2 colloids and (b)
absorption spectra of TiO2 colloids deducting the solvent effect. |
|
In addition, it is notable
that the photon energy corresponding to the two inflection points (see the arrow in Figure
3b) is same as the energy of the two pronounced peaks at 260nm and 310nm respectively in
the absorption spectra. Moreover, their sites in the absorption spectra do not show
difference even for the different samples. Henglein [7] suggested that the
maxima which appeared in the absorption spectrum of very small particles (Figure 3a)
corresponded to the optical transition to the first excited state in quantized particles
of different size and thus to the "magic"
agglomeration numbers existed in the size distribution of the sample. This suggests that
two agglomeration numbers exist in our colloids, which produce two kinds of primary
particle corresponding to two peaks in optical absorption. With the decrease of the H+/Ti
ratio, the particles including larger agglomeration number increase so that the contents
of peak at 260nm decrease.
3.3 Fluorescence spectra
Fluorescence spectra recorded in colloidal samples are presented in Figure 4. The emission
increases sharply after 330nm and reaches a maximum at 340nm. A broad and weak peak
appears at 370nm. The spectra extend to 500nm. The relation of the relative content of the
two peaks is same as the results of the absorption spectra.
Fluorescence has been described as transitions from surface states.
Among the three crystalline polymorphs: rutile, anatase and brookite, the nature and
spectral position of anatase have not come to a common conclusion and agreement. Broad
asymmetric UV emission at 375nm (Dlfwhm=100nm) has been observed in m m-size
anatase colloids [8]. In recent publication [9], more complex
fluorescence spectra of TiO2 colloidal nanoparticles in the range between 300
and 500nm have been observed.
As one can see that only in UV range, two fluorescence continual peaks
have been observed in our samples. It is reasonable to assign the UV emission spectrum
observed at 370nm in the present study to interband transitions. The excited state of TiO2
can be considered as Ti3+...O- and the
emission may be due to electron exchange leading to Ti4+. Another fluorescence
of TiO2 at 340nm has not been reported. In many hydroxides, a fluorescence peak
at 340nm usually appears if 280nm excitation is used, while this continuum disappears in
corresponding oxides. So we suggest that the fluorescence at 340nm results from the
transition of surface Ti-OH bond.
Since emission is mostly a particle surface phenomenon, differences in
emission intensities must originate from differences in surface properties of the
particles. In figure 4, the decrease of the content of peak at 340nm indicates that the
surface hydroxy groups decrease with the increase of the particle size. Comparatively, the
peak intensity at 340nm is stronger than that of T-25 powder, suggesting that the Ti-OH
groups are rich in our colloids.

Fig.4 Fluorescence spectra (solid lines) and
fitted-cruves (dashed lines) of TiO2 colloids
3.4 Quantum mechanical calculations
Quantum mechanical calculations of the band gap shift in small semiconductor particles
were first made by Efros [10] and Brus [11, 12]. Brus has described
the analytical formula based on effective mass approximation (me, h=const):
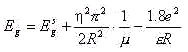
where Eg and Egs are the
particle and solid-state band gap energies; R is radius of the particle; m -1=me*-1+mh*-1 is
the reduced mass of the exciton, here me* is the effective mass of
the electron and mh* is the effective mass of the hole; e is dielectric constant of the material. Another approach based on
quantum mechanical variation calculation has been described by Kayanuma:[13]
In this paper, the dielectric constant e =12[14] and the electron and hole masses of 10me
and 0.8me[14], respectively (where me is a free electron
mass), have been used. The calculated calibration curves are presented in Figure 5. Anpo's results [4] (+ ) and our
experimental point ( with solvent effect and n deducting solvent effect) are given in Figure 5. The calculated
results are used to compare with our results of AFM and Anpo's
results. Experimental results with solvent effect deviate to the calculations by Kayanuma
and Brus. After excluding the effect of solvent, our experimental results are in agreement
with theoretical predictions by Brus (dashed line), though they deviate to the calculation
described by Kayanuma (solid line).
| Fig.5 Calibration curves lonset(R): according to the Kayanuma's
variational calculation (solid line); according to Brus formula (dashed line); Anpo's result (+) and our experimental point (. with solvent effect and n deducting solvent effect). |
4. CONCLUSION
A small and stable TiO2 sol can be obtained by peptizing commercial TiO2
powder. The particle size can be effectively controlled by acidity of the peptized
solution. The smallest particle size is 1.2nm in H+/Ti=1.2 measured by AFM.
Small TiO2 particles show typical Q-size effects. The onset of UV-vis
absorption shifts to shorter wavelengths with the decrease of the particle size, and the
exciton energy becomes pronounced. A fluorescence peak appeared at 340nm is assigned to
the transition of surface Ti-OH groups. Moreover, their intensities are consistent with
size distribution. In addition, our results are in agreement with the quantum mechanical
calculations by Brus.
ACKNOWLEDGMENT
We are also with to express our thanks to Dr. Xiaohui Qiu for AFM experimental
assistance.
REFERENCES
[1] (a) Norris J R, Meisel D. Photochemical Energy Conversion. New York: Elsevier,
1989; (b) Serpone N, Pelizzetti E. Photocatalysis Fundamentals and Applications. New
York: Wiley, 1989.
[2] (a) Schiavello M. Photocatalysis and Environment Trends and Applications, London:
Dordrecht, 1987; (b) Ollis D F, Al-Ekabi H. Photocatalytic Purification and
Treatment of Water and Air, New York: Elsevier, 1993.
[3] Kormann C, Bahnemann D W, Haffmann M R. J. Phys. Chem., 1988, 92: 5196.
[4] Anpo M, Shima T, Kodame S, Kubokawa Y. J. Phys. Chem., 1987, 91: 4305.
[5] Monticone S, Tufen R, Kanaev A V. Chem. Phys. Lett., 1998, 295: 237.
[6] Zhang Y H, Wu M, Xiong G X et al. Chinese J. Catal. (Cuihua Xuebao), 1999, 20 (3):
305.
[7] Henglein A. Chem. Rev., 1989, 89: 1861.
[8] Chandasekaran K, Thomas J K. J. Chem. Soc., Faraday Trans., 1984, 80: 1163.
[9] Serpone N, Lawless D, Khairutdinov R. J. Phys. Chem., 1995, 99: 16646.
[10] Efros Al L, Efros A L. Sov. Phys.Semicond. (Engl. Transl.), 1982, 16: 772.
[11] Brus L E. J. Chem. Phys., 1984, 80: 4403.
[12] Brus L E. J. Phys. Chem., 1986, 90: 2555.
[13] Kayanuma Y. Solid State Commun., 1986, 59: 405.
[14] Enright B, Fitzimauric D. J. Phys. Chem., 1996, 100: 1027.