http://www.chemistrymag.org/cji/2000/023018re.htm |
|
--- The formation, microstructure and application of the third phase
Fu Xun, Hu Zhengshui ,Wang Debao, Liu Huan, Hu Xiaopeng
(Qingdao Institute of Chem. Tech., Shandong 266042, China)
Received
Feb. 14, 2000; Supported by the National Natural Science Foundation of China (No. 29971020).Abstract Recent advances in the study of three-phase
extraction systems have been reviewed. The results are emphasized on the third phase
formation, extraction mechanism, and application of the newly formed phase.
Keywords Extraction, The third phase
In liquid-liquid extraction system, the problem of
three-phase (i.e., two organic phases and one aqueous phase) can be encountered. The
formation of the third phase might be reported first by Healy et al[1]. The
third phase (or the middle phase, the heavy organic phase) interferes with the extraction
process. Many authors have applied themselves determining when the third phase forms and
knowing how to avoid its formation since 60's[2-5].
Srinivasan et al[6] reported that there was a Limiting Organic Concentration
(LOC, when the metal loading concentration in the organic phase is higher than that value,
the organic phase will split into two parts, heavy and light organic solutions) and that
they measured the values for tri-alkyl phosphates diluent / Pu(IV), U(VI), Th(IV) HNO3
system. A review of third phase formation in extraction of actinides by neutral
organophosphorus extractants was made by Vasudeva Rao(7). The results indicated
that all experimental conditions, such as aqueous acidities, diluents, extractant
structure and extracted metals, can affect the third phase formation. Das et al[8] reported
the three-phase behavior discovered in the ammonium and amine extractant systems, such as
tri-octyl amine, Aliquat336. The methods of avoiding the third phase formation usually
used are raising extraction temperature, adding modifiers and keeping the metal loading
concentration below LOC[9,10].
Since the 80's, several achievements in the studies of extraction
processes from the viewpoint of interfacial chemistry have been reported[11].
Wu et al[12] testified that reversed micelle or w/o microemulsion formed in the
organic phase; Wang et al[13] proved that micelle or o/w microemulsion formed
in the aqueous phase of the extraction system; and Osseo Asare[14] indicated
that the third phase in an extraction system could be analogue to a middle phase
microemulsion in a typical surfactant system. But, general speaking, studies on the
three-phase extraction are lacking, the results emphasized on the distribution of metals
between the three phases, the extraction mechanism, microstructure of the third phase,
especially on the application of the newly formed phase are lacking. The recent advances
in the studies of three-phase extraction systems will be reviewed in this paper.
1. THREE-PHASE BEHAVIOR AND THE EXTRACTION MECHANISM
1.1 Acidic extractant system
Organo-phosphoric acid could be taken as a representative of the acidic extractants.
The pseudo triangular phase diagram was for the first time used by Paatero et al[15]
in studying the phase behavior of extraction system of Cynaex272 - n-hexane / NaOH aqueous
solution. There were distinguished mono-phase microemulsion, two-phase, three-phase and
liquid crystal regions in the phase diagram. The result indicated that the highest
solubilization of water in the organic solution was found at NaA/HA=2:1. The author then
purified Cyanex272 to di-(2,4,4-trimethylpentyl) phosphinic acid (HDTMPP), and
investigated the effect of modifier TOPO (tri-octylphosphoric oxide) on the phase behavior
of the above system[16]. The results showed that the sodium salt of HDTMPP
(NaDTMPP) could be considered as an anionic surfactant, and that both impurities and
modifiers in it could affect its amphiphilic properties, then influence the phase behavior
of the extraction system. One of the most obvious changes was that three-phase region
became smaller in the presence of the modifier TOPO, but there was no detailed study on
the newly formed middle phase.
 |
|
 |
Fig.1 Phase behavior of HDTMPP-kerosene
/ H2O-NaOH-Na2SO4 system at 298K. (taken from ref.16) (a) CHA(i)=0.4mol/L, Na2SO4 0.1mol/L; (b) a=0.75, Na2SO4 0.1mol/L; (c) CHA(i)=0.4mol/L, a=0.75 |
Hu et al[17] measured the
three-phase behavior of HDTMPP-kerosene / H2O-NaOH-Na2SO4
system. The effects of the initial concentration of the extractant ( CHA,i), of
the saponified fraction of HDTMPP (a=CNaA/CHA,i ), and of the
salinity of the aqueous solution on the phase volume were shown in Fig.1a, b and c
respectively. These figures directly perceived the third phase formation and disappearance
and the volumes of each phase. The salinity scanning (Fig.1c) was very similar to the
result observed in the anionic surfactant system, i.e., the microemulsion changed from
Winsor type I, through type III, to type II. The experiment also confirmed that the third
phase disappeared when a little octanol or tributyl phosphate (TBP) was added.
1.2Neutral extractant system
Fu et al[18]measured the three-phase behavior of TBP-kerosene / H2SO4-H2O
system in a very wide concentration range, and found that the third phase formed when the
equilibrium H2SO4 concentration in the bottom phase (CH2SO4,b)
ranged from 6.8 to about 16 mol/L regardless of the initial TBP contents (10-60%,v/v) in
the organic solution (see Fig.2), and that the composition of the middle phase (in terms
of TBP, H2SO4 and H2O) was only a function of CH2SO4,b.
They proposed two extraction mechanisms for H2SO4 and H2O
into the third phase in different CH2SO4,b:
[1] Extraction part (CH2SO4,b=6.8-10.6 mol/L), where H2SO4
and H2O are transferred into the third phase mainly by complex conversion from
(TBP)2·H2SO4·(H2O)3 (written as P1)
to TBP·H2SO4·H2O (written as P2);
[2] Solubilization part (CH2SO4,b=10.6-16 mol/L), where excess H2SO4
( beyond TBP:H2SO4=1:1) and H2O are extracted into
the third phase owing to the solubilization by the aggregates of the complex of TBP:H2SO4=1:1.
| Fig.2 Plot of phase volume vs. CH2SO4,b for the TBP-kerosene/ H2SO4-H2O extraction system at 298K, TBP, 10-60%. (taken from ref.17) |
|
Fig.3 Phase diagram of the TOA·HCl-heptane-H2O system at 298K. (taken from ref. 19) |
They also investigated the
three-phase extraction of TBP-kerosene / H2SO4-TiOSO4
(0.2mol/L) and compared it with above H2SO4 extraction system[19].
In the metal system, the composition of the third phase (in terms of TBP, H2SO4,
Ti(IV) and H2O) was a function of CH2SO4,b in the range of 6.3-10.2
mol/L. The extraction of Ti(IV) hardly occurred in the two-phase region, but extraction
ratio increased rapidly after the third phase formed. It was attributed to the
co-aggregates formed by TBP·TiOSO4·(H2O)4
(written as P3) with available TBP:H2SO4=1:1 complexes.
1.3Ammonium and amine extractant system
Considering tri-octyl amine as a representative, Fu et al[20] prepared the pure
salt of TOA·HCl, and proposed the triangular phase diagram of
TOA·HCl-heptane-H2O system at 298K (see Fig.3). The
phase behavior was controlled by the amphiphilic properties of the salt, like a cationic
surfactant.
Most phase boundaries were straight lines.
The salt-rich phases (including liquid crystal phase and heavy organic phase) presented a
typical lamellar structure owing to the self-assembly of the salt molecules. The
solubilization capacities of water in the polar layers and that of heptane in the apolar
layers were within the values of TOA·HCl : H2O :
heptane = 1:2:3 (molar ratio).
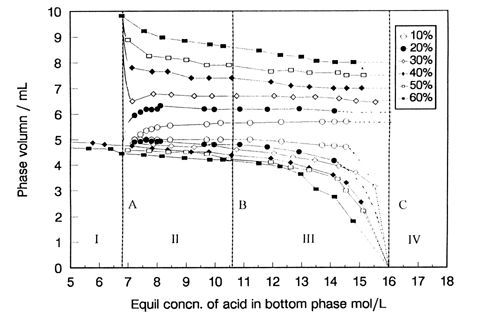
Fig.4 Phase behavior of the extraction systems TOA-kerosene / HCl (ZnCl2
or FeCl3) at 298K. (taken from ref. 20)
a, acid system; b, Zn2+ system; c, Fe3+ system.
The authors[21] then
measured the phase behavior of TOA in heptane (0.98 mol/L)/HCl (0 - 10 mol/L) system, and
indicated that the third phase formed after CHCl,i > 0.1 mol/L. The
composition analysis for the middle phase (in terms of TOA, TOA·HCl,
heptane and H2O) showed that the solubilization of water and heptane were less
than the above molar ratio in the range of CHCl,i= 0 - 1.0 mol/L, i.e., the
neutralized fraction of TOA by HCl a<1. It was attributed to
the effect of free TOA on the polarity of the salt aggregates. The investigation also
showed that the phase behavior changed when kerosene was used as the solvent or metal ion
such as Zn2+ or Fe3+ was added in the HCl aqueous solution (see
Fig.4). The distribution data of metals indicated that the metal was extracted mainly into
the middle phase, and the metal content in the light organic phase could be negligible.
2.THE MICROSTRUCTURE OF THE MIDDLE PHASE
Acidic extractants (saponified), extractants containing nitrogen (salted), and neutral
extractants can be analogues to the anionic, cationic, and non-ionic surfactants
respectively, so can be the third phase in an extraction system to the middle phase
microemulsion in a typical surfactant system. It is well known that the middle phase has a
bi-contineuous structure, but no detailed information of the structure of the third phase
formed in an extraction system has been found in the literature. We have chosen many
samples of the third phase to observe their microstructures by the TEM technique after the
freeze-fracture replication.
The images of the middle phase in TOA-heptane / HCl-water extraction system are shown in
Fig.5 and those in TBP-kerosene / H2SO4-TiOSO4 are shown
in Fig.6. Their typical lamellar structures imply that the packing parameter of the
amphiphilic molecules in the extraction systems is about unity, i.e., R=V/al equals about
one (where V represents the molecular volume, a the section area of the polar head,
and l the length of the hydrophobic
group).
|
|
|
|
| Fig.5
TEM images of the salt-rich phases in the system of TOA·HCl -heptane-water. (taken from
ref. 19) a, b, c and d are for samples of T1, T2, T3 and T5 in Fig.3 respectively. |
|||
|
|
|
|
| Fig.6
TEM images of the middle phases in the system of TBP-kerosene / H2SO4-
TiOSO4. (taken from ref. 18) (a) x50K, CH2SO4,b=7.0, CTi,m=0.01 mol/L; (b) x50K, CH2SO4,b=8.2, CTi,m=0.12 mol/L; (c) x50K, CH2SO4,b=9.4, CTi,m=0.21 mol/L; (d) x50K, CH2SO4,b=11.0, CTi,m=0.21 mol/L |
|||
|
|
|
| Fig.7
TEM images of the middle phases in the system of TBP-kerosene / H2SO4-
H2O. (taken from ref. 17) (a) x100K, CH2SO4,b=7.6, CH2SO4,m=1.6 mol/L; (b) x100K, CH2SO4,b=10.4, CH2SO4,m=2.7 mol/L; (c) x100K, CH2SO4,b=13.8, CH2SO4,m=6.3 mol/L |
||
The
images of the middle phases in TBP-kerosene / H2SO4-water extraction
system are shown in Fig.7. The sample 2 was taken from the boundary B (CH2SO4,b=10.4
mol/L, CH2SO4,m=2.7 mol/L, see Fig.2), where the extracted complex was mainly
in the form of TBP·H2SO4·H2O, and it showed a typical lamellar structure owing to
the complex assembly. Samples 1 and 3 were chosen from regions II and III respectively.
Their TEM images indicated that both the existing complex composed of TBP:H2SO4
>1:1 and solubilized H2SO4 and water could weaken the lamellar
structure. It seems that the microstructure of the third phase went through a loose state
tight state loose state process with the increase of CH2SO4,b in the
three-phase region, and it can be illustrated by the transition of microemulsion of the
Winsor types II![]() III
III![]() I with the increase of
aqueous acidity.
I with the increase of
aqueous acidity.
3. APPLICATION OF THE THIRD PHASE
Two aspects of studying the applications of the newly formed phase have been reported
in the literature. Firstly, the purification method for acidic organo-phosphoric
extractants was established based on the principle of concentration effect of the
amphiphilic compounds in the middle microemulsion. Hu et al[22] reported that
P204, P507 and Cyanex272 could be purified by striping the third phase formed in the
extractant-gasoline / NaOH-Na2SO4 equilibrium system. Compared with
the copper salt recrystallization method, this new method has many advantages such as
simplicity, high recovery, high purity and less cost.
Secondly, some nanosized ultrafine powders were prepared by direct
precipitating the loaded metal in the middle phase. Yang et al[23] prepared ZrO2
sized 10 nm with high purity by precipitating the metal from the third phase formed in
TBP-kerosene / mineral acid-Zr(IV) extraction system. The features of this method are
that, the micro-environment similar to reversed micelles can confine the growth of
particles; the amphiphilic species including extractant and its ion-pair with metal can
prevent particle agglomeration; and the separation effect of the extraction process can
ensure the high purity.
Our laboratory investigated the preparation of ultrafine powder of TiO2
using the middle phase formed in the TBP-kerosene / H2SO4-TiOSO4
extraction system.[19] The results indicated that the amorphous precipitate
changed to anatase crystal after calcination at 600℃, and
changed to rutile crystal after calcination at 1200℃. The
anatase powder had a narrow size distribution of 20 nm, but the high temperature
calcination would make the particles agglomerated. It is worth indicating that a rather
higher metal content in the organic solution than any other methods, such as alkoxide
hydrolysis, sol-gel method, and microemulsion method, promises the production of ultrafine
powder on a large scale.
4. CONCLUSIONS
4.1 Acidic extractants (saponified), extractants
containing nitrogen (salted), and neutral extractants can be analogues to the anionic,
cationic and non-ionic surfactants respectively. The phase behavior of an extraction
system is controlled by the amphiphilic properties of the extractant or extracted species.
The microstructure of the third phase depends on the packing parameter of the amphiphilic
molecules, R=V/al. The middle phases of the systems TBP/H2SO4
(metal) and TOA/HCl (metal) have lamellar structure.
4.2 The process from formation to disappearance of the third phase can be
analogous to the transition process of a surfactant microemulsion system from Winsor type
II, through type III to type I. The extraction mechanism in the three-phase region may be
different to that in the two-phase system. In two-phase region, the transfer of metal to
the organic solution by a complex reaction, but in the three-phase region, the
solubilization must be considered. Metal is extracted mainly into the third phase, and the
metal loading will change the phase behavior.
4.3 The artificially prepared third phase can be used in purification of
extractants and production of ultrafine powders.
4.4 We suggest paying more attention to the study of three-phase
extraction systems on their phase behavior, extraction mechanism, especially on the
application of the newly formed third phase.
REFERENCES
[1] Healy T V, Mckay H A C. Trans. Farady Soc., 1956, 52: 633.
[2] Ochhkin A V. Fiz. Khm., 1960, 54: 1862.
[3] Kertes A S, Mckay H A C, Healy T V et al. Solvent Extraction Chemistry of
Metals. Clevelland: CRC press, 1967.
[4] Hanson C, Patal A N. J. Appl. Chem., 1969, 19: 20.
[5] Irving H, Edgington D N. JINC, 1959, 10: 306.
[6] Srinivasan T G, Vijayasaradhi S, Dhamoduran R et al. Solvent Extraction and
Ion Exchange, 1998, 16 (4): 1001.
[7] Vasudeva R P R. Solvent Extraction Ion Exchange, 1996, 14 (6):
955.
[8] Das N R, Lahiri S. Sep. Tech., 1994, (5): 791.
[9] Blumberg R. Liquid-Liquid Extraction. London: Academic Press, 1988.
[10] Xu G, Wang W, Wu J et al. Principle of Extraction Chemistry. Shanghai: Science
and Technology Press of Shanghai, 1988.
[11] Wu J, Shi N, Zhou W et al. Proceedings of the Second Conference of
Solvent Extraction of China, Shanghai, 1991, 7.
[12] Wu J, Gao H, Chen D et al. Science in China B (Zhongguo Kexue), 1980, 23
(12): 1533.
[13] Wang D , Wu J , Li Y et al. Science in China B (Zhongguo Kexue), 1995, 38 (5): 449.
[14] Osseo-Asare K. Advances in Colloid and Interface, 1991, 37: 123.
[15] Paatero E, Ernola P, Sjoblom J et al. ISEC'88, Moscow,
1988, 124.
[16] Paatero E, Lantto T, Ernola P. Solvent Extraction Ion Exchange, 1990, 8 (3):
371.
[17] Hu Z, Xin H, Pan Y et al. Applied Chemistry (Yingyong Huaxue), 1995, 12
(5): 10.
[18] Fu X, Hu X, Hu Z et al. Colloids and Surfaces, 1999, 152: 335.
[19] Hu Z, Hu X, Cui W et al. Colloids and Surfaces, 1999, 155: 383.
[20] Fu X, Liu H, Xue M et al. Eng. Chem. & Met. (Huagong Yejin), 1999, 20
(1): 5.
[21] Fu X, Liu H, Li Z et al. Solvent Extraction Ion Exchange, 1999, 17 (5):
1281.
[22] Hu Z, Pan Y, Ma W et al. Solvent Extraction Ion Exchange, 1995, 13 (5):
965.
[23] Yang C, Hong B, Chen J. Powder Tech., 1996, 89: 149.