http://www.chemistrymag.org/cji/2000/025023ne.htm |
|
Shen Xingcan, Liang Hong, Jiang
Zhiliang, He Xiwen#, Shen Panwen #
(Institute of Bioinorganic Chemistry, Department of Chemistry, Guangxi Normal University,
Guilin 541004,China #Department of Chemistry,
Nankai University, Tianjin 300071,China)
Received Feb. 10, 2000; Supported by the National Natural Science Foundation of China (NO. 29961001) and the Foundation for Talents Striding across the Century of Guangxi.
Abstract The binding equilibrium between
I- and human serum albumin (HSA) or bovine serum albumin (BSA) has been studied
by resonance light-scattering (RLS), fluorescence spectrometry and equilibrium dialysis.
The enhancement of RLS were founded as the I- binding to the HSA or BSA, while
the fluorescence quenching at 350 and 700 nm. From the equilibrium dialysis results, the
binding of I- to HSA or BSA fits to a phase distribution model instead of the
Scatchard model, and the order of magnitude of phase distribution was found to be 104.
The dialysis at different pH indicates that the binding mechanism is probably due to the
electrostatic forces between the I- and protonated amino-acid residues.
Keywords serum albumin, iodine, equilibrium dialysis,
resonance light-scattering, phase distribution
1. INTRODUCTION
Iodine is one of living element, and is also called intellect element [1]. The
serum albumin, which constitutes 60% of blood plasma, has very important physiological
functions. It is, therefore, important to know the detail of I- interactions
with serum albumin, The metal ions, little molecules and medicines etc interacting with
HSA or BSA have been reported [2-5], but there is no reports about behavior of
I- binding to HSA or BSA. The RLS [6], which is a new method put
forward recently, has rarely been studied in bioinorganic chemistry and photochemical
study of HSA and BSA [7]. The investigations of I- binding to HSA or
BSA with RLS, fluorescence spectrometry and equilibrium dialysis are helpful for us to
understand absorbing, conveyance, etc physiological function of iodine. Our study
indicates that RLS is a sensitive way in bioinorganic chemical study besides ultraviolet
and fluorescence spectra.
2. EXPERIMENTAL
Electrophoretic pure HSA and BSA were purchased from Sino-American Biotechnology Company
Beijing Branch and mailed freshly. All solutions were prepared with analytical grade
reagents and double deionized water. The RLS data were obtained by scanning the excitation
and emission monochromators (Dl =0.0 nm) with a shimadzu RF-540 spectrofluorophotometer (Kyoto
Japan), within the wavelength region from 200 nm to 1000 nm. The excitation and emission
slits were set to 10 nm. With lex=296 nm, scanning the emission wavelength region from
200 nm to 1000 nm, the fluorescence spectra of HSA or BSA binding with I- were
obtained. Hitachi UV-3400 spectrophotometer was employed for the concentration of HSA and
BSA [2]. The steps of equilibrium dialysis were described in ref. [2,5], but
were performed in the darkness. The concentration of KI solution before dialysis was
titrated with iodimetry, and the concentration of free I- ions ([I-]) after dialysis was obtained by spectrophotometry [8].
3. RESULTS AND DISCUSSION
3.1 RLS and fluorescence spectra
Fig.1 shows the RLS spectra of I- binding
to HSA. The spectral characteristics of BSA are similar to that of HSA [7].
There are five characteristic peaks in the RLS of HSA or BSA, two strong intensity peaks
at about 296 nm and 470 nm, a broad peak at about 690 nm, two shoulder peaks at 590 nm and
900 nm respectively. The intensities of the RLS (IRLS ) are enhanced
greatly after I-
binding to HSA. The IRLS is directly proportional to the
ratio of [I-]/[HSA] in the range of 0-120
at the most sensitive wavelength 296 nm. The concentration of HSA is 1×10-5 mol / L.
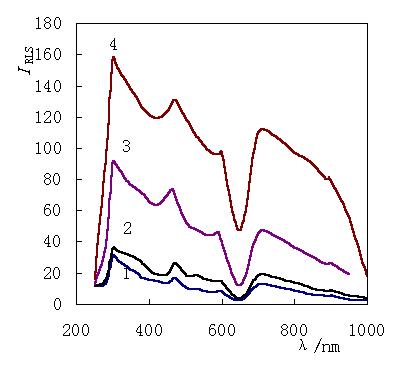 |
Fig.1 The resonance
light-scattering (RLS) of HSA 1:HSA; 2:[I-]/[HSA]=5:1; 3:[I-]/[HSA]=50:1; 4:[I-]/[HSA]=100:1. The concentration of HSA is 1×10-5 mol / L. |
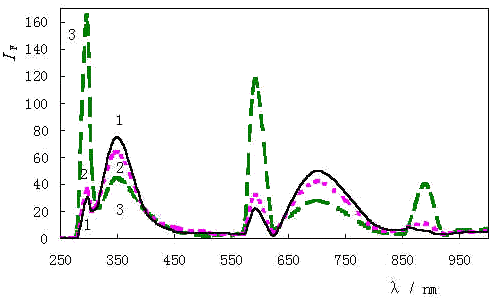 |
Fig.2 Fluorescence spectra 1:HSA; 2:[I-]/[HSA]=5:1; 3:[I-]/[HSA]=50:1. The concentration of HSA is 1×10-5 mol / L. |
The fluorescence spectra are shown in Fig.2. The
intensity of fluorescence spectra (IF ) was quenched greatly at
350 nm and 700 nm which are the first and second fluorescence emission peaks respectively.[7]
The quenching results from the "heave atom effect" of iodine[9].
Whereas the behavior of the peaks at 296 nm, 592 nm and 888 nm are different from
fluorescence emission peaks at 350 nm and 700 nm: as I- binding to HSA they
increase instead quench. The three peaks are the first, second, and third resonance
scatter peak [7], and their behavior and mechanism is similar to that of RLS [10].
These peaks ignored in fluorescence spectra are of significance to find
out the information of resonance scattering.
 |
Fig.3 The concentrations of BSA a:1×10-4; c: 2×10-4 mol/ L; The concentrations of HSA b:1×10-4; d: 2×10-4mol/ L. At pH7.43 |
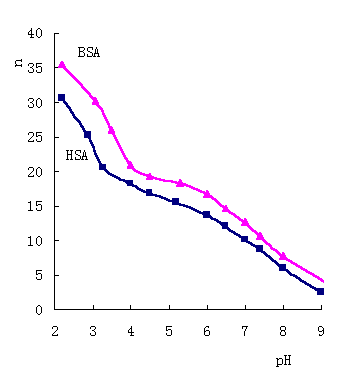 |
Fig.4 The concentrations of BSA and HSA 1×10-5 mol/ L |
3.2 Equilibrium dialysis study
The results of equilibrium dialysis at pH7.43 are shown in Fig.3. By plotting ![]() vs [I-],
straight lines are obtained, which is not similar to the Cu2+, Mn2+
etc binding to HSA and BSA [2,5]. The ratio
vs [I-],
straight lines are obtained, which is not similar to the Cu2+, Mn2+
etc binding to HSA and BSA [2,5]. The ratio ![]() /[I-] (
/[I-] ( ![]() is the
average binding number [2]) is practically constants, so the binding of I- here does not seem appropriate for the Scatchard model which has been
widely employed to the combination of small ions or molecules with proteins [4,5,11].
Instead a phase distribution model allows a good quantitative treatment of the
experimental findings. So the HSA and BSA considered as microphase distinct from the
aqueous solution in which it is dispersed. Thus I- is distributed between the
albumin microphase and aqueous solution. The phenomenon is singular [3]. The
distributed constant coefficient kdc of I- binding to HSA and BSA are 2.01×104 L/ mol and 2.31×104 L / mol respectively.
is the
average binding number [2]) is practically constants, so the binding of I- here does not seem appropriate for the Scatchard model which has been
widely employed to the combination of small ions or molecules with proteins [4,5,11].
Instead a phase distribution model allows a good quantitative treatment of the
experimental findings. So the HSA and BSA considered as microphase distinct from the
aqueous solution in which it is dispersed. Thus I- is distributed between the
albumin microphase and aqueous solution. The phenomenon is singular [3]. The
distributed constant coefficient kdc of I- binding to HSA and BSA are 2.01×104 L/ mol and 2.31×104 L / mol respectively.
3.3 The pH effect and binding mechanism
Series of equilibrium dialysis experiments of I- binding
to HSA and BSA at different pH are investigated, and the result is shown in Fig. 4. The
curves of ![]() (I-) vs pH are found there very similar to the curve [12]
of
(I-) vs pH are found there very similar to the curve [12]
of ![]() (H+) vs pH. The isoelectric point of HSA or BSA is at pH4.7 [13]. As pH<4, the lower pH
is, the more H+ ions bind to serum albumin, and the more positive charge the
albumin molecules own [12]. Form the Fig.4, the number of binding I - increases fast as pH<4. On other hand, when pH>6,
the higher pH is, the fewer H+ ions bind to serum albumin, and the more
negative charge the albumin molecules show, and the fewer I -
ions bind. In the range of pH 4-6, the two curves change mildly. The results imply that
the number of H+ binding to amino-acid residues at albumin molecules has an
effect to the binding of I-. As a result that the
distributed constant coefficient kdc is change with pH. It is concluded that I - ions combine to the protonated basic amino-acid residues
with electrostatic energy. The basic amino-acid residues such as: Lys, His, Arg and
amino-group of N-terminal. The opposite charges at albumin molecules surface are
neutralized as the binding of I -, and the protective
water layer is destroyed as the distribution of I-
ions between the two phases. As a result the macromolecules of HSA or BSA most possibly
trend to aggregating and forming colloid in big size, so the intensity of RLS is enhanced
considerably.
(H+) vs pH. The isoelectric point of HSA or BSA is at pH4.7 [13]. As pH<4, the lower pH
is, the more H+ ions bind to serum albumin, and the more positive charge the
albumin molecules own [12]. Form the Fig.4, the number of binding I - increases fast as pH<4. On other hand, when pH>6,
the higher pH is, the fewer H+ ions bind to serum albumin, and the more
negative charge the albumin molecules show, and the fewer I -
ions bind. In the range of pH 4-6, the two curves change mildly. The results imply that
the number of H+ binding to amino-acid residues at albumin molecules has an
effect to the binding of I-. As a result that the
distributed constant coefficient kdc is change with pH. It is concluded that I - ions combine to the protonated basic amino-acid residues
with electrostatic energy. The basic amino-acid residues such as: Lys, His, Arg and
amino-group of N-terminal. The opposite charges at albumin molecules surface are
neutralized as the binding of I -, and the protective
water layer is destroyed as the distribution of I-
ions between the two phases. As a result the macromolecules of HSA or BSA most possibly
trend to aggregating and forming colloid in big size, so the intensity of RLS is enhanced
considerably.
The binding of I- to HSA and BSA
appropriate to the phase distribution model and related physiological effect need further
studies.
[1] Li Y. Iodine, Life and health (Dian, Shengming, JianKang). Beijing: Science Press, 1998: 4-8.
[2] Liang H, Xing B G, Wang X J et al. Chinese Sci. Bull, 1998, 43 (5): 404-408.
[3] Plsavento M, Profumo A. Talanta, 1991, 38 (10): 1099-1106.
[4] Zhang B L, Wang W Q, Yuan R Y. Acta Chimica Sinica (Huaxue Xuebao), 1994, 52: 1208-1212.
[5] Liang H, Tu C Q, Zhang H Z, Shen X C et al. Chinese Journal of Chemistry (Zhongguo Huaxue), 2000, 18 (1): 35-41.
[6] Pasternack R F, Collings P J. Science, 1995, 269: 935-939.
[7] Liang H, Shen X C, Jiang Z L et al. Chinese Chemistry Letter (Zhongguo Huaxue Kuaibao), 2000, 11 (3): 251-254.
[8] Marczenko Z (author), Zheng Yong-xi et al (author of translation). Spectrophotometric Determination of Elements, Beijing: Geological Press, 1976: 257-260.
[9] Zheng G Z, Huang X Z et al. Fluorescence Analysis (Yingguang Fenxi). 2nd edition, Beijing: Science Press, 1990: 92-93.
[10] Liu S P, Liu Q, Liu Z F et al. Analytica Chimica Acta, 1999, 379: 53-61.
[11] Scatchard G, Scheinberg I H. J. Am. Chem. Soc., 1950, 72: 535-540.
[12] Wang K. The Bioinorganic Chemistry (Shengwu Wuji Huaxue), Beijing: Qinghua University Press, 1988, 216.
[13] Putnam F W. The plasma protein structure, functions and genetic control. New York: Academic Press, 1975: 133-172.