http://www.chemistrymag.org/cji/2000/02b050ne.htm |
Nov. 18, 2000 Vol.2 No.11 P.50 Copyright |
Sun Xiaohong, Liu Yuanfa*, Yang Xuwu*, Gao
Shengli*
(Chemical Research Institute of Northwest University, Xi'an 710069, China; *Department of
Chemistry, Northwest University, Xi'an 710069, China)
Received Apr. 25, 2000; Supported by the National Natural Scientific Foundation of China (No. 29871023). Supported by the Educational Committee of Shannxi Province (No. 99JK09).
Abstract The synthesis for new compound
4-amino-6-tert-butyl-3-methylthio-1,2,4-triazin-5-(4H)-one sulfate is reported. The
product was confirmed by elementary analysis, IR and 1H NMR. The combustion
energy has been determined by Rotating-bomb calorimeter. Biological tests showed that it
has herbicidal activities corresponding to the Metribuzin.
Keywords 4-amino-6-tert-butyl-3-methylthio-1,2,4-triazin-5-(4H)-one sulfate,
synthesis, standard enthalpy of formation, biological activity
1 INTRODUCTION
4-amino-6-tert-butyl-3-methylthio-1,2,4-triazin-5-(4H)-one (Metrubuzin) is known as a
broad-spectrum, high effect and low toxicity herbicide. It is used for controlling many
types of weeds in corn, soybean and sugarcane fields [1-2]. While Metribuzin
sulfate has not reported in the literature. The compound was first synthesized and the
structure was also confirmed.
Present report provides a method for the preparation of Metribuzin
sulfate. In acid medium, using methyl sulfuric acid as methylating agent, selective
S-methylation, high yield of Metribuzin sulfate can be obtained. The product has been
confirmed by elementary analysis, IR and 1H NMR. The combustion energy has been
determined by Rotation-bomb calorimeter. Its standard enthalpy of formation was
calculated. Biological activity has been tested by small-cup method. Results showed that
the Metribuzin sulfate is more stable than Metribuzin; the biological activities are
similar to each other. These provide the theoretical basis for further research of
Metribuzin sulfate on its properties and application.
2 EXPERIMENTAL
2.1 Equipments and reagents
XRC-1 melting point apparatus (uncorrected), Perkin-Elmer 2400 elementary analysis
instrument, EQUINOX55 Infrared spectrometer (KBr disks), FX-90Q NMR spectrometer and RBC-1
Rotating-bomb calorimeter were used for the measurement.
4-amino-6-tert-butyl-3-mercapto-1,2,4-triazin-5-(4H)-one (trizine) was
prepared by literature procedure[3], recrystallization twice from methanol.
m.p.212°C-214°C(Lit. [3]
m.p.212°C-214°C). All reagents used are A.R. grade.
2.2 Experimental procedure
40g (0.2mol) of trizine in 90% H2SO4, the reaction mixture was
subsequently stirred, heated until all the solid dissolved. Methanol 10ml (0.3mol) was
added dropwise. The reaction temperature was kept at 60°C-70°C. After the mixture stirred for 2h-3h, the reaction
mixture was cooled and poured into ice-water. The Metribuzin sulfate as white crystal was
collected, yield>95%. Recrystallizaton from ethanol.
3 RESULTS AND DISCUSSION
3.1 Analysis results
The melting point of the Metribuzin sulfate is 218°C-219°C. Elementary
analysis (%): C 30.80(30.77), H 5.15(5.13), N 18.00(17.95) . So, the composition of the
Metribuzin sulfate is C8H16N4O5S2
M.W. 312. IR (cm-1): 2750-3500 (m, NH4SO4), 2970 (CH3),
1730 (C=O), 1639 (C=N), 1361,1218 (C-N) , 1066,679 (C-S). 1H NMR (TMS,
DMSO-d6):δ1.35 (9H, s, C(CH3)3),δ2.48
(3H, s, SCH3),δ9.20 (4H, s, -NH4SO4). Fig.1 is the 1H
NMR of Metribuzin sulfate. Because the -NH2 and the H2SO4 formed
-NH4SO4, this decreased the electric density around the N atom, the
peak moved to downfield in δ9.20. Fig.2 is the 1H NMR of Metribuzin
sulfate, D2O as solvent. No peak for protons of -NH2 group can be
found in Fig.2. Because these protons are substituted by the deuterium atoms of D2O.
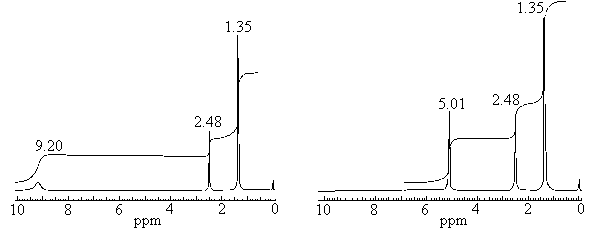
Fig.1 1H NMR spectra of Metribuzin sulfate Fig.2 1H NMR spectra of Metribuzin sulfate
(added D2O) Using acidimeteric method, the content of H2SO4 was determined. The result was Metrabuzin : H2SO4 = 1 : 1(mol).
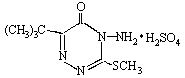
All the analytical results indicates that the structure of the Metribuzin sulfate is as bellow:
3.2 The standard enthalpy of formations of Metribuzin sulfate and Metribuzin
Same method of reference[4] was used to determine the combustion energy of the sample,DcU = (- 15909±15.05) J.g-1 . The standard combustion enthalpy of the sample, DcH0 , refers to the combustion enthalpy changes of the following ideal combustion reaction at 298.15K and 101.325kPa:
C8H16N4O5S2(s) + 23/2 O2(g) = 8CO2(g) + 2SO2 (g) + 2N2(g) + 8H2O(l)
The standard combustion enthalpy of the Metribuzin sulfate was calculated from the combustion energy using the following equation: DcH0 = DcU +DnRT, DcH0 = (-4964.46±4.70) kJ.mol-1. According to the above thermochemical equation, standard enthalpy of formation,
DfH0, was calculated by Hess's law. The result of the calculation was DfH0 = (-1063.88±4.83) kJ.mol-1.
The standard combustion enthalpy of the Metribuzin was determined by the same method as DcH0 = (- 5294.85±3.50) kJ.mol-1. The standard enthalpy of formations of the Metribuzin was DfH0 = (- 150.85±3.66) kJ.mol-1.
The results indicate that the Metribuzin sulfate is more stable than the Metribuzin. It is very useful for the storage of the pesticide.
3.3 The biological activity test
Small cup method was used and the same gemmation barnyard grass seeds were selected for the test. Distilled water was used as contrast. The concentration of both Metribuzin sulfate and Metribuzin was 2.5%(W/V) in ethanol solution and then diluted to 25 and 50ppm. The temperature was kept at 25°C±3°C under daylight lamp for 72 hrs. The heights of the grass were measured. According to the following equation, the inhibition percentage of the growth Dh% were calculated.
Dh% =
Where, h0 and h refer to the height of the sample and the contrast. The results showed: the heights of the grass using Metribuzin were 13.30% (25ppm), 28.42% (50ppm); the Metribuzin sulfate were 13.21% (25ppm) and 28.40% (50ppm). Biological tests showed that it has herbicidal activities corresponding to the Metribuzin.
REFERENCES
[1] Zhang Dianjin, Chen Renlin. Encyclopedia of Chemical Weeds Control on
Farmland. Shanghai: Shanghai scientific and technological Literature Publishing House,
1994: 625-626.
[2] Wang Yu, Chen Tiebao, Huang Chongyan. Pesticide (Nongyao), 1998, 37 (4):
27-28.
[3] Herbert K, Werner S, Wolfgang L. US Patent,1979, 4 175 188.
[4] Gao Shengli, Yang Xuwu, Ren Dehou et al. Thermochimica Acta, 1996, 287: 177-182.