http://www.chemistrymag.org/cji/2000/02b052ne.htm |
Nov. 28, 2000 Vol.2 No.11 P.52 Copyright |
Effects and mechanism of magnetic field on the form and structure of phosphate
Ma Wei, Guo Liyan, Liu Xuehu, Yu Jie
(Dalian Univ. of Tech., Dalian 116024)
Received March14, 2000; Supported by NSFC (29777011)
Abstract The magnetic effects on the
structure and crystal form of calcium phosphate and magnesium phosphate were studied by
means of chemical analysis, microscopy, X-ray diffraction (XRD) and scanning electron
microscopy (SEM). The results showed that the heterogeneity was produced and crystal form
was very small in the magnetic field. The conductivity and surface tension of the solution
decreased. The consistence of phosphorus increased 2.2 times higher than that without
magnetic field. The magnetic field effect was more evident on weak acid. The results
indicate that the magnetic field modified the dissociation equilibrium of phosphate ions
and the nature of the charge on the surface of the crystal. Therefore the combined
chemical and physical effects could not make the large and regular crystal of phosphate
grow. It could be used to remove or prevent the scale formation.
Keywords magnetic field, phosphate, and crystallization
1. INTRODUCTION
Application of the magnetic field on preventing and removing scales in circulating water
has developed for 40 years and it has attracted much attention for its good effect [1-4].
In recent years, the magnetic field was increasing applied in industry to prevent and
remove scale of calcium carbonate. Ronald Gehr has studied the SO42-
contained solution under the magnetic field of 4.75T and the results showed that the
magnetic field benefit forming of gypsum so that scales can be prevented and removed
easily[2]. H.E.Lunbager.Madsen [5] considered that the magnetic
field only had effects on the carbonate and phosphate with diamagnetic metal ions during
his research on the crystal of some inorganic salts. Also good results have been obtained
in treating scales of sugar medium with electromagnetic field. Several hypotheses about
the mechanism have been raised, such as changes of the water structure or the magnetic
particles in the solution. But an integrated theoretical system of the magnetic field
effects hasn’t formed yet till now.
In recent years, the rich nutrition phenomenon of the water system has become more and
more serious, which do the phosphate detergents, phosphate water treatment agent and the
synthetic fertilizer cause. The composition of the remaining agent would cause the second
pollution when phosphate-dispersing agent is used to solve the problem [6,7].
So researchers have shown great interest in the physical methods applying magnet, light,
microwave and so on to treat the polluted water [6-8]. We just studied the
problem of phosphate scales through the Nd-Fe-B magnetic field and tried to make a further
explanation of the mechanism of magnetic field effects according to a series of
experiments.
2. EXPERIMENTAL
2.1 Materials
A.R grade CaCl2, (NH4)3PO4 and
KH2PO4 were used in the experiment.
2.2 Main Instruments
The main instruments we used were the magnetizing equipment, spectrometer model 721,
X-ray diffraction model X/Dmax-III A (made in Japan), scanning electron
microscope model S-510 (made in Japan), microscope model L2000, conductometer model
DDS-11A and the surface stagnation equipment.
2.3 Methods
When the solution of CaCl2, or MgCl2 (NH4)3PO4
and KH2PO4 is passed through the magnetic field of certain intensity
(0.4T) with the speed of 2.0mL/s, the changes of the conductivity and the surface tension
against the solution without magnetic field are determined.
When the equally mixed solution of magnetically treated CaCl2
(0.5mol/L) or MgCl2 and (NH4)3PO4 (0.1mol/L)
is passsed the magnetic field of 0.4T, the crystal growth is observed under microscope.
The content of phosphorus was analyzed and the results were compared with that of without
magnetic field after 48 hours.
Five groups of data were needed to take the mean value and the shapes and structures of
the crystals were tested through XRD and SEM after filtration.
3. RESULTS
3.1 The crystallization process and the structure of the phosphate
A large quantity of tiny crystals were separated after the magnetic treated solutions
are mixed up. The crystals were uniform and an obvious layer of crystal water was around
each of them (just like the ice). As time passing, the particles mostly remained small but
a few had gathered to form groups. When we observed the same process of the solution
without magnetic field, we found the crystallization speed slower and the particles
larger. The large particles were black under the microscope. The results of the further
test by SEM showed that the size of the particles with magnetic field treated was smaller
(showed in Fig1.).

a (×6000)
b (×3000)
Fig.1 SEM photographs of calcium phosphate (a-with magnetic field, b-without
magnetic field)
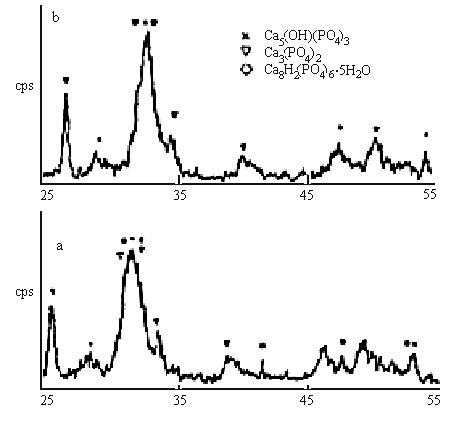
2q/(°)
Fig.2 XRD pattern of calcium phosphate (a-with magnetic field, b-without magnetic
field)
The structures of the crystals were analyzed by XRD. The results (in Fig.2) indicate that the existence of Ca8H2(PO4)6.5H2O ( OCP) except Ca5(OH)(PO4)3 (HAP) and Ca3(PO4)2 (TCP) for the magnetic treated particles, but OCP does not exist in the particles without magnetic treatment. This indicates that the magnetic effects are favourable to the formation of OCP.

a (×6000)
b (×3000)
Fig.3 SEM of the magnesium phosphate (a---with magnetic field, b---without magnetic
field)
We have also studied the sedimentation process of magnesium phosphate by the same method. The result of SEM was showed in Fig.3, which is similar to Fig.2.
3.2 Effects of magnetic field on the physical
and chemical parameters of the solution
The magnetic effects on the physical and chemical parameters were studied by passing the
solution through the magnetic field and the results showed some properties of the solution
changed after magnetic treatment. The changes of surface tension and conductivity are
showed in tab1.
Tab.1 Changes of surface tension and conductivity with magnetic field
| solution (mol/L) |
Conductivity (ms/cm×103) | Surface tension (N/m×10-2) | ||||
| Without m. | With m. * | change rate | Without m. | With m. | change rate | |
| KH2PO4 (0.58) | 0.77 | 0.85 | +10.39% | 71.51 | 69.05 | -3.45% |
| CaCl2 (0.05) | 7.80 | 7.80 | 0 | 72.75 | 67.82 | -6.77% |
| (NH4)3PO4(0.5) | 8.50 | 9.05 | +6.47% | 73.98 | 69.05 | -6.67% |
* m. --abbreviation of magnetic field
4. DISCUSSIONS
Surface tension is an important physical property of solution. In terms of thermodynamics,
the surface contraction of solution Gibbon's energy is a spontaneous
process of the decrease in the system. So, a certain quantity of energy was needed to form
the new surface. The solution with magnetic field separated out a large quantity of tiny
crystals in a short time just because of the decrease of surface tension, which is showed
in table1. The conductivity can reflect the concentration and state of electronic
particles in the solution. The magnetic field had no obvious effect on the conductivity of
CaCl2 while it increased the conductivity of phosphate according to table1. The
effects of magnetic field on the ionization of weak electrolyte were clearly proved.
Phosphoric acid is polyprotic undergoes stepwise dissociation as given
by the following equilibrium:
H3PO4=H2PO4-+H+ [H+][H2PO4-]=K1[H3PO4] ----(1)
H2PO4-=HPO42-+H+ [H+][HPO42-]=K2 [H2PO4-] ----(2)
HPO42-=PO43-+H+ [H+][PO43-]=K3[HPO42-] ----(3)
The values of ionization constants are reported as follows: K1=7.5×10-3, K2=6.2×10-8, K3=2×10-13. Thus depending on the [H+], different species like H3PO4, H2PO4-, HPO42- and PO43- may exist in the solution. The mass balance on the phosphate as follows:
CT = [H3PO4]+[H2PO4-]+[HPO42-]+[PO43-] ----(4)
Where CT is the total
concentration of the phosphate. According to our experiments, CT of the mixed
solution was 80mg/L with magnetic treatment of 48hrs, which was almost 2.2 times higher
than that without magnetic field. The latter was 35mg/L when other conditions ware
identical.
It can be seen from equation (1)-(3) that major proton transfer from weak acid to water.
M.L.Mikhdson and Z.Kolloidn[7] published that the Lorentz force on charged
particles should have considered. which is given by the vector product:
F=qv×B ----(5)
Where F is the Lorentz
force (N); q the charge of particles(C); v the velocity (m/s) and B
the magnetic induction (T). M.L.Mikhelson thought that the
Lorentz force caused by magnetic field on moving ions in solution is far too weak to
influence the crystallization process by Simple calculation [4]. But
H.E.Lunbager.Madsen discovered that the magnetic field really had some effects on the
diamagnetic particles in spite of no obvious effects on the paramagnetic particles through
his research on the inorganic salt crystallization process [5]. The results of
our experiments showed that magnetic field effects on the weak electrolyte were more
evident than that on the strong electrolyte. So, the magnetic effects are determined not
only by the magnetic properties but also by the state of the particles in the solution.
The effects on the diamagnetic positive ion and the negative ion of weak acid and weak
base were more evident. Just as the effects on the phosphate, the magnetic field promoted
the solubility because the phosphoric acid was weak acid and undergoes stepwise
dissociation. The polyphone resulted in the gathering of tiny particles against the
shaping of large regular crystals. (Showed in Fig.1 and Fig.2). Kelvin effect improved the
deliquescent performance of the particles.
In addition, the Lorentz force probably effected the surface electric
charge distribution of the crystal cores (electric particles) when they passed through the
magnetic field. So there were some changes on the crystal surface and the diffuse layer
between the crystal and the solution, which would result in the changes of the shape and
structure. The changes were beneficial to removing and preventing of the scales.
5. CONCLUSIONS
The effect of magnetic field on the crystallization process prompt the separation of tiny
particles and Kelvin effect increases the solubility of the phosphate.
The effects of magnetic field have close relations to the properties of
both the magnetic field and the particles in the solution. The effects on the diamagnetic
positive ion and the negative ion of weak acid and weak base are more evident and result
in the difference of the products.
The difference of the water ring around the crystals show the change of
the electric charge distribution on the crystal surface. Mechanism studies and
experimental further verification remained to be carried on.
[1] Donaldson J, Grimes S. New Scientist, 1988, 18 (4): 43.
[2] Ronald G et al. Wat. Res., 1995, 3 (29): 933.
[3] Ahamad H M, Dixit S G. Wat. Res., 1992, 6 (66): 845.
[4] Mikhelson M L, Kolloidn Z. Chem. Absstr., 1977, 87: 577.
[5] Lundager H E, Madsen. Journal of Growth, 1995, 152: 94.
[6] Safarik I. Wat. Res., 1995, 29 (1): 101.
[7] Yasozo Y S, Takashi F. Wat. Res., 1994, 28 (5): 1175.
[8] Broomberg. J. Magneic and Electrical Separation, 1999, 3 (9): 169.