http://www.chemistrymag.org/cji/2001/034014pe.htm |
Apr. 1,
2001 Vol.3 No.4 P.14 Copyright |
Heterogeneous photocatalytic degradation of methylene blue aqueous solution under the coexistence of metalloporphyrin polymer and air
An Taicheng, Gao Jinzhang#, Chen Hui#, Zhu Xihai( #Department of Chemistry, Northwest Normal University, Lanzhou, 730070; School of Chemistry and Chemical Engineering, Zhongshan University, Guangzhou, 510275, China.)
Received Sep. 21, 2000; Supported by the National Natural Science Foundation of China (No.29977030) and Natural Science Foundation of Gansu (No. zs-981-A24-058-Y)
Abstract High pressure mercury lamp (HPML) was used for irradiation, the kinetics of photocatalytic degradation of methylene blue (MB) in water with air sparging has been investigated in the presence of Polymeric[Co(II)meso-Tetra(4,4'-Biphenylbisulfony)-phenyl porphyrin]. Chemical Oxygen Demand (COD) proximately dropped by half, with the decolourizing efficiency being about 50-60% after 8 hours. The changes of the visible spectra and the kinetics curve of reaction were measured. It was observed that photocatalytic degradation of MB obey pseudo first-order reaction with the apparent first-order decay constant k= 0.3544 h-1, half life 1.96 h. Compared with HPML, natural sunlight (NSL) had similar effect on the photocatalytic degradation of MB. The effects of a variety of factors such as pH, irradiation sources and initial concentration of MB were discussed. MB in water could degrad within 3 h in the presence of metalloporphyrin polymer and diluted solution of hydrogen peroxide.Keywords Photocatalytic degradation, Methylene blue, Metalloporphyrin, Kinetics
The issue of treatment of organic pollutants
in water is an ever increasingly global problem because many organic contaminants are
inevitable byproducts of industry and agriculture. In 1980s, the total production of
synthesized dyes alone was more than 800 thousand tons per year. In the course of
production and utilization, a significant amount of dye-containing wastewater was
discharged into receiving water.
In recent years, several alternative oxidative degradation processes
using catalytic and photochemical methods have been developed. Considerable academic
interest has been focused on the photocatalytic degradation of contaminants in water with
semiconductor TiO2. There have been many reports on the photocatalytic
oxidation of organic pollutants such as dyes, pesticides, surfactants etc. in water [1-5]. During
last two decades, there were also a few reports on the catalytic oxidation of organic
substrate in non-aqueous solvent using metalloporphyrin as photocatalysts[6-9]
. But, this is the first report on the heterogeneous photocatalytic degradation of organic
pollutants in water using suspension metalloporphyrin polymer and irradiated by HPML.
Moreover, the process could also be driven by NSL, significantly potential application of
solar energy considering the photosensitivity of metalloporphyrin.
1.1 Materials
Polymeric Co[meso-Tetra (4,4'-Biphenylbisulfony)phenyl-porphyrin] (Co[P(TBPSOPP)]) was prepared, purified and characterized as previously reported [10], and visible spectra of polymeric ligand exhibits a soret band at 422.1 nm, with the Q bands at 524.2, 558.7, 599.0, and 658.2 nm. The soret band varied little and the Q bands decreased from 5 to 2 with the ligand coordinating with metals. MB was obtained from Chmond Rd. (London) and used without further purification. The tanked oxygen was used and air was sparged with an air pump. The other reagents were analytical grade and all the solutions were prepared with double-distilled water.
1.2 Recommended procedure
5 mg of Co[P(TBPSOPP)] was added into a quartz double-layered reactor (self-designed) containing 35 ml of 3.74 mg/L MB solution, then stirred with 78HW-1 magnetic stirrer prior to irradiation with 450 W HPML. With air sparging, photocatalytic degradation took place in the reactor. Water was recycled in a 501 model thermostatic bath and the temperature of the reactor kept at 20°C except for the temperature experiments. The absorbance of the sample solution were determined on model UV-754 spectrophotometer at 664 nm with glass cells (10 mm optical path length) every hour after standing for 5 minutes. Experiments performed under NSL were carried out in August and September in Lanzhou, Gansu, China (East longitude 103°53' and North latitude 36°03').
1.3 Calibration curve
A calibration curve was obtained for MB and found to be linear in the range of 0.75-7.5 mg/L. The MB concentrations and absorbances were well correlated with the regression equation as follows:
A=0.0139 +0.1439×C (R = 0.9979)
where A is the absorbance of the MB; C is the concentration of MB (mg/L) and R is the regression coefficient for the straight line. 2. RESULTS
2.1 The kinetics of photocatalytic degradation of MB solution
In the presence of Co[P(TBPSOPP)], the change of the visible spectra and the kinetics of photocatalysis of MB were investigated at pH 6.6. The contrast degradation curve of MB is shown in Fig. 1. From the relevant data, it can be seen that MB dramatically decrease with the increase of irradiation time . It was found that the logarithmic values of the concentrations of MB solution was presented in Fig.2 as a straight line with the increase of irradiation time. This indicates that the photocatalytic degradation of MB obeyed pseudo first-order kinetics. The rate equation can be represented as follows: lnCt = lnC0 -kt
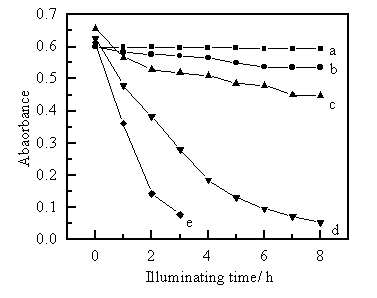
a: No catalyst and in the dark;
b: Absorption under 5mg catalyst and no illumination.
c: No catalyst plus HPML;
d: Absorption under 5 mg catalyst , HPML plus air pumping;
e: Absorption under 5 mg catalyst, HPML, air pumping plus H2O2.
Compared with HPML, NSL
had similar effect on the photocatalytic degradation of MB. The process also conformed the
pseudo first-order kinetics.( Fig.2 ) The kinetics regression equations were shown below
respectively:
lnCt= 1.5639-0.3544t (R = -0.9974), life time t1/2=1.96 h. (HPML)
lnCt= 1.4888-0.2598t (R =
-0.9878), life time t1/2=2.67 h. (NSL)
From above, we can concluded that HPML has better effect than NSL
because that HPML has fast degradation rate than NSL under the same conditions.
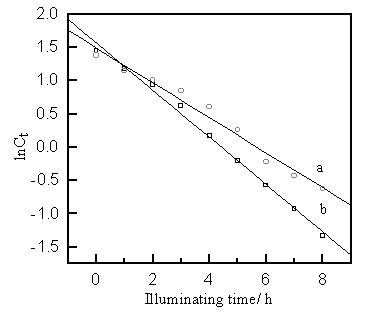
a: NSL; b: 450 W HPML.
2.2 Determination of COD
COD was determined according to the reported method[11]. The
contrast COD concentrations of working sample and measurement accuracy are listed in Table
1. It can be found that decreasing rate of COD, on the average, reached 47.2% after 8
hours photocatalytic degradation process. This may be due to the complete decomposition of
MB.
COD (mg/L) |
confidence |
SD |
Degradation |
|
Before photocatalytic degradation |
49.4 40.1 |
43.9±4.2 |
|
|
After photocatalytic degradation |
27.1 22.2 |
23.2±3.3 |
|
|
2.3 Determination of decolourization
rate
After 8 hours photocatalytic degradation, the solution of MB became colorless. The
chromaticity and decolorization rates of the dyestuff in the aqueous solution were
calculated according to the equations described previously[12].
Eleven points were selected at 20 nm intervals to measure their
absorbance in the visible spectrum band (420-620 nm). The
decolourization rate and the relative chromaticity were determined from the absorbance of
the sample solution and Pt-Co standard chromaticity under the selected eleven wavelengths.
The changes of chromaticity of MB in the sample solution are shown in
Table 2. It is well known that MB can be bleached under NSL without any catalyst, however,
from Table 2, it is just 29.6% of MB was self-bleached with the degradation rate of about
34.9%. In the presence of metalloporphyrin, though HPML has higher degradation rate than
NSL, it was evident that the decolouring efficiency under the irradiation of NSL was much
higher than HPML, presumably due to the excitation by stronger emission lines of NSL than
HPML in the visual spectrum band.
420 |
440 |
460 |
480 |
500 |
520 |
540 |
560 |
580 |
600 |
620 |
chromaticity |
|
Pt-Co standard * |
0.272 |
0.215 |
0.250 |
0.217 |
0.156 |
0.093 |
0.047 |
0.020 |
0.009 |
0.005 |
0.003 |
500° |
initial
|
0.002 |
0.004 |
0.012 |
0.020 |
0.024 |
0.028 |
0.046 |
0.081 |
0.146 |
0.257 |
0.337 |
371° |
ZSL self-sensitivity |
0.011 |
0.013 |
0.016 |
0.022 |
0.026 |
0.030 |
0.035 |
0.053 |
0.096 |
0.149 |
0.221 |
261° |
degraded sample |
0.033 |
0.035 |
0.034 |
0.034 |
0.033 |
0.032 |
0.035 |
0.038 |
0.042 |
0.045 |
0.047 |
158° |
NSL degradation |
0.014 |
0.011 |
0.013 |
0.012 |
0.011 |
0.010 |
0.013 |
0.016 |
0.021 |
0.026 |
0.029 |
68° |
*The absorbance of Pt-Co standard chromaticity was determined with a model 721 spectrophotometer [12]
.2.4 Recovery of photocatalyst
The used photocatalyst was recovered and measured after twice reaction. It could be
observed that photocatalytic activity of the catalyst reduced with the irradiation time.
The activity of the recovered photocatalyst in the first recovery time was higher than the
second time, which indicated that the photocatalyst obtained in the solution could be
recovered to some extent for further photo-degradation.
3.1 The contrast disappearance of MB
Results of the photo-decomposition of MB in various conditions are presented in Fig. 1. It was shown that the concentrations of MB decreased with the increase of irradiation time. From curve (a) of Fig. 1 the absorbance did not vary at all and the slope is plat. It is well known that MB can be absorbed onto solid easily, but from curve (b), only a small amount of MB could be absorbed onto photocatalyst without irradiation. Only 10 per cent of MB could be photodegraded when illuminated with HPML in the absence of metalloporphyrin in curve (c), although MB have been used as a sensitizer in previous reports [13,14] for the photocatalytic degradation of organic contaminants in water. In curve (d), with added photocatalyst and illuminated with HPML, MB decreased rapidly and photocatalytically degraded completely within 8 hours with air sparging. The significantly greater slope was obtained for curve (e). The degradation rate greatly speeded up, and the decomposition of MB completely finished within 3 hours in the presence of 2.5×10-2 mol/L hydrogen peroxide.
3.2 Effect of pH
The effect of pH was studied in the pH range of 2.90-12.00 by adjusting pH with diluted sodium hydroxide or hydrochloride acid solution. The results shown in Fig. 3 indicate that the effect of pH on photocatalytic degradation rate was noticeable. The degradation rates of MB increase distinctly with the increase of the pH value.
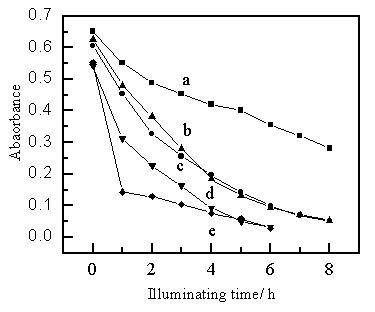
a: pH 2.9; b: pH 4.2; c: pH 6.6; d: pH 9.3; e: pH 12.0.
3.3 Effect of working temperature
In order to analogize actual water supply, the temperatures of the photocatalytic
degradation were measured from 293 to 313 K. Their kinetic parameters were shown in Table
3. It can be seen that the effect of temperature on the decomposition rates is not
noticeable. Thus the working temperature of photocatalytic degradation should be chosen
according to the temperature of ambient dye-containing water.
Table 3. The kinetic parameters of temperature effect
| The temperature(K) | Kinetic equation |
kinetic constant k (h-1 ) |
Half time t1/2 (h) |
293 |
lnCt=1.5644-0.3544t |
0.3544 |
1.96 |
303 |
lnCt=1.3331-0.3591t |
0.3591 |
1.93 |
313 |
lnCt=1.3576-0.3646t |
0.3646 |
1.90 |
3.4 Effect of oxidants
During the course of photocatalytic degradation, the effects of various oxidants were
measured. Their first-order kinetic curves are shown in Fig. 4. The effects of oxidants
were significant when small concentration of oxidants were added. The HO· radicals play an important role in the catalytic oxidation reaction
of dyes. The HO· was produced easily by the illumination of hydrogen
peroxide [15,16], so the hydrogen peroxide was added to investigate its action. The
MB was completely degraded in the presence of 2.5×10-2 mol/L of hydrogen peroxide within 3 hours (curve c of Fig. 4 ), compared with 8
hours under air-saturated atmosphere (curve a ) and oxygen-saturated atmosphere (curve b
).
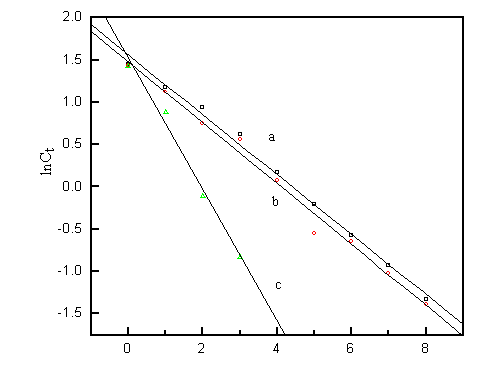
a: Air-Sparge; b: Oxygen-sparge; c: H2O2 plus air-sparge.
3.5 Effect of initial concentration
of MB
According to the recommended procedure, the photocatalytic degradation was discussed by
changing the initial concentration of MB solution from 2.05 to 6.75 mg/L. It was observed
that initial concentration of MB has a significant effect on the degradation rate and it
decreases with the increase of the initial concentration. The contrast effect was shown
for MB in Table 4. In this experiment, the optimum initial MB concentration of 4.25 mg/L
was chosen to conduct the experiment.
Table 4 the kinetic parameters in various initial concentration of MB
| Initial Concentration C0 (mg/L) | 6.658 |
5.442 |
4.253 |
2.085 |
The percentage of degradation |
71.4% |
80.6% |
91.7% |
86.6% |
lnC0 ( real value ) |
1.8345 |
1.7608 |
1.5638 |
0.7352 |
lnC0 ( calculated value ) |
1.8950 |
1.6941 |
1.4480 |
0.7351 |
Decay kinetic constant k (h-1 ) |
0.1713 |
0.2016 |
0.3544 |
0.4054 |
Half time t1/2 (h) |
4.91 |
3.44 |
1.96 |
1.71 |
3.6 Effect of amount of photocatalyst
In this procedure, 2, 5, 7 mg of metalloporphyrin were used respectively. The curves of
phototcatalytic degradation were also discussed. It is obvious that the amount of
photocatalyst has a little effect on the degradation rate. Fast initial rate was obtained
when more catalyst was added, but the same result was observed within 8 hrs.
3.7 Effect of different illuminants
It is well known that the emission spectra of HPML mainly focus on the ultraviolet band
having the advantages of short-wave emission and large energy to initiate photochemistry
reactions of chemicals. However, the spectrum of NSL is ranged from ultraviolet to near
Infrared and with the main flux concentrated on the visible light band. In order to
investigate the practical application of this technology in ambient water supply, NSL was
chosen to use as the source of irradiation. It was found that for HPML, it is evident that
the higher flux of illumination is, the larger photo-degradation rate is obtained.
Compared with HPML, NSL had similar effect on MB. It can be concluded that this experiment
could also be repeated with NSL. The potential usage of sun light in water treatment is
indicated by these results.
MB aqueous solution can be degraded with irradiation with both HPML and NSL in the presence of Co[P(TBPSOPP)]. The degradation of MB in water obeys pseudo first-order reaction.
Photocatalyst can be deposited and recycled without second pollution.
After 8 hrs photocatalytic degradation, the dyestuff (MB) can be completely decolourized with the solution becoming colorless; the COD concentrations of dyestuff also dropped apparently.
The photocatalytic degradation of MB in water could be greatly accelerated in the presence of hydrogen peroxide. REFERENCES
[1] Matihews P W. Wat. Res., 1991, 25 (10): 1169.
[2] Wang Q Q, Jiang W C, Bao Y Y et al. Environment pollution & control, 1992, 14 (1): 2.
[3] Ollis D F, Pelizzetti E, Serpone N. Environ. Sci. & Technol., 1991, 25 (9): 1523.
[4] Wang Q Q, Jiang W C. (Huanjing Kexue yu Jishu), 1994, 66 (3): 1.
[5] Matthews R W. Pure & Appl, 1992, 64 (9): 1285.
[6] Polo E, Amadelli R, Carassiti V et al. Inorg. Chim. Acta, 1992, 192: 1.
[7] Chen H, An T C, Fang Y J et al. J. Mol. Catal. A: Chem., 1999, 147 (1-2): 165.
[8] Chen H, An T C, Fang Y J et al. Indian J. Chem., B: 1999, 38 (7): 185.
[9] Maldotti A, Bartocci C et al. Inorg.Chem., 1996, 35: 1126-1131.
[10] Wan R M, Li S B, Wang Y P et al. J.Appl.Polym.Sci., 1998, 67: 2027.
[11] EPA of China. The Methods for Water and Wastewater monitoring & Analysis (3rd edition), Beijing: China Environmental Technolge & Science Press, 1989, 354.
[12] Zhou J J. Electroplating and Environmental Protection, 1988, 8 (2): 35.
[13] Acher A, Fischer E, Zellingher R et al. Wat. Res. 1990, 24 (7): 837.
[14] Li X Z. Technology of water treatment, 1984, 10 (1): 7.
[15] Fujihira M et al. Bull. Chem. Soc. Jpn., 1982, 55: 666.
[16] Wei H B, T Li, Yan X S. Advance Environ. Sci., 1994 , 2: 50.