http://www.chemistrymag.org/cji/2001/033013ne.htm |
Mar. 1,
2001 Vol.3 No.3 P.13 Copyright |
The synthesis and crystal structure of 5,5'-bis(azidomethyl)-3,3' -bis(1,2,4-oxadiazole)
Li Zhanxiong, Ou,Yuxiang, Chen Boren
(School of Chemical Engineering and Material Sciences, Beijing Institute of Technology, Beijing, 100081,China)
Received Sep. 20, 2000
Abstract 5,5'-bis(azidomethyl)-3,3' -bis(1,2,4-oxadiazole) was synthesized and characterized by elemental analysis, IR, 1H NMR and MS as an energetic compound. The mechanism of the rearrangement of 1,2,5-oxadiazole to form a 1,2,4-oxadiazole system was studied. The crystal structure of the title compound was determined by X-ray diffraction.Keywords synthesize, crystal structure, furazan, isofurazan
N. D. Zelinsky Institute of Organic Chemistry
have been studying 1,2,5-oxadiazole(furazan) for about two decades[1,2]. They
found that aromaticity of the furazan ring usually increased the thermal stability of its
derivatives, and the planarity of the ring provided them with high density. In 1965, R. A.
Olofson and J. S. Michelman pointed out that the furazan ring system was an aromatic
heterocycle[3]. The weakness of the N-O bond in furazan was suggested by an
analysis of its mass spectrums. It agrees with the results of the resonance forms of
furazan , which is calculated by E. Saegebrath [4].
In 1977, N. Vivona found that when N-C=S sequences were present in the
side-chain of the phenylthioureido derivatives, the systems may rearrange and new
five-member heterocycles may be obtained with 1,2.4-oxadiazole(isofurazan) system.[5]
this must be ascribed to the superior nucleophilic properties of the sulphur atom in
attacking the nitrogen atom of furazan ring.
In this work, the N-C=O sequence was introduced into the 3-position of
furazan and an isofurazan system was obtained.
1 SYNTHESIS
General information. Virtually compound II is potentially dangerous high
energetic explosives and should be handled carefully. Melting points (uncorrected) were
measured on a XT4A micro-meldometer and elemental analysis on a heraeus CHN-RAPID
instrument.IR spectra were recorded on a MAGANT-560, MS on a KYKY-5#, and 1H
NMR on a ARX400MHz NMR spectrometers (TMS as internal standard) respectively.
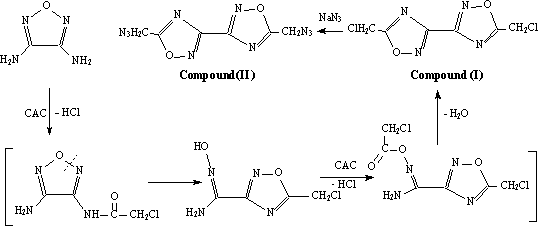
Treatment of 3,4-diaminofurazan(DAF) with chloroacetyl chloride (CAC)
can give 5,5'-bis(chloromethyl)-3,3'
-bis(1,2,4-oxadiazole) (compound I) which is deemed to rearrange from 1,2,5-oxadiazole
(scheme 1). Then compound I was treated with sodium azide to give 5,5'-bis(azidomethyl)-3,3' -bis(1,2,4-oxadiazole) (compound II).
The structure of compound I and II were identified by elemental
analysis, IR, 1H NMR, MS(FAB), and the crystallography shows that compound II
is a bis(isofurazan) derivatives ulteriorly.
Compound I. (procedure a) from DAF. Chloroacetic chloride 5.4g
(48mmol) was added slowly to a solution of 1.2g(12mmol) DAF in DMF (10mL) at 0°C. This mixture was stirred for 1h at
0°C and for 2h at room temperature. Pouring the reaction mixture onto 60g of crushed ice
/water, the white solid was collected and recrystallized from 15mL of acetone/water(1:1)
giving 1.99g (67.7%) of the products. M.p.= 100~101°C.IR(KBr): 2987(m), 1574(s), 1210(s), 1178, 906(s), 782 cm-1. C6H4N4O2Cl2
: cal.C30.64,H1.70,N23.83. Found:C30.66,H2.49,N23.80. 1H NMR(acetone-d6):δ5.06 (4H,-CH2-).
(procedure b) from diaminoglyoxime(DAG). The procedure of this way is
same to (a) basically. Treatment DAG with CAC at 40°C give 1.60g (54.4%) of the product.
IR(KBr): 2989,1569(s),1214(s),1175,904(s),780cm-1. C6H4N4O2Cl2:
cal. C30.64,H1.70, N23.83. Found: C30.68,H2.45,N23.62. 1H NMR(acetone-d6):δ5.06 (4H,-CH2-).
Scheme 1
Compound II. 0.65g
(10mmol) of sodium azide in 4mL of water was added drop wise with stirring to a solution
of 1.25g (5mmol) of compound I in 30mL of acetone. The mixture was refluxed for 4h and
then poured into ice/water (150mL). The precipitate was collected and recrystallized from
12mL of acetone/water(1:1) to yield 0.71g (56.3%) of the product. M.p.= 67°C. IR(KBr):
2998,2160(s),2125(s),1565(s)1207(s),964,906,772 cm-1.C6H4N10O2:
cal.C29.03 H1.61, N56.45.
found: C29.75,H1.37,
N57.24. 1H NMR(acetone-d6):δ4.97(4H,-CH2-). MS(FAB) (M+1)+=249(28%).
Treatment of DAF with CAC could introduce N-C=O sequence into the
3-position of furazan and the rearrangement took place. This gave an isofurazan ring and
an oximido group. Then the oximido acted on CAC to give a chloroacetoxy group. As the
result of dehydrating of chloroacetoxy and the adjacent amino group, another isofurazan
ring formed. In this way we obtained compound I.
To confirm the cyclizing mechanism of chloroacetoxy and the adjacent
amino group of procedure (a), we selected diaminoglyoxime(DAG) to react with CAC . The
oximido group of DAG could react with CAC to give a chloroacetoxy sequence with an
adjacent amino group. In this way, we got the same compound I followed by treatment with
sodium azide to give compound II.
Compound II was identified as a bis(isofurazan) derivatives with low
hydrogen content and low melting point. It may be used as energetic additives.
2 STRUCTURE OF COMPOND II
2.1 Diffraction data collection of compound II
The product was dissolved in a acetone water mixture (1:1) and the solvents were
evaporated slowly to obtain a colorless single crystal with size of 0.60×0.20×0.08mm. It
was used for X-ray diffraction analysis.
The diffraction analysis was carried out on Siemens P4 diffractometer
and the graphite monochromator with radiation MoK\α( l=0.071 073 nm) was used. The scan type 2q-w, q range for data collection was 2.09 to
24.99 degree All 1007 reflections were collected, 869 Independent reflections [I>2s(I)] were used for
structure refinement.
The crystal structure was solved by direct method. All atomic
coordinates and equivalent isotropic thermal parameters of non-hydrogen atoms were refined
by full-matrix least-squares method with R indices (all data) R1 = 0.0395, wR2 = 0.0766,
Final R indices [I>2s(I)] R1 = 0.0286, wR2 = 0.0703.
2.2 Structure refinement
The empirical formula of compound II is C6H4N10O2
, formula weight 248.19. Crystal system belongs to Triclinic,Space group P 1. a=0.858 18(2)nm, b=1.143 36(4)nm, c=1.432 75(5) nm. Z=4, Volume 2.4953(9) nm3, dc=1.652
kg/m3.
Figure 1 and figure 2 show the molecular
configuration and cell packing respectively. Atomic coordinates
and equivalent isotropic thermal parameters of non-hydrogen atoms are listed in table1 and
table2 and the Anisotropic displacement parameters in table 3.
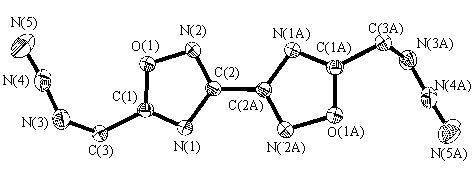
Fig 1 Molecular configuration of compound II
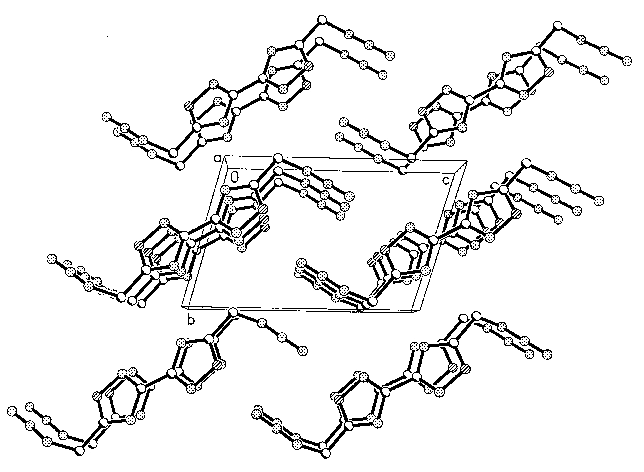
Fig 2 Cell packing of compound II
Table 1. Atomic coordinates and equivalent isotropic thermal parameters of non-hydrogen atoms
| atoms | X×104 | Y×104 |
Z×104 |
Ueq×105/ nm2 |
| O(1) N(1) N(2) N(3) N(4) N(5) C(1) C(2) C(3) |
2799(3) |
6111(2) |
7688(1) |
47(1) |
Table 2. Bond lengths [nm] and angles [deg]
| bond | bond length |
bond |
bond angle |
O(1)-C(1) |
0.133
9(2) |
C(1)-O(1)-N(2) |
106.41(10)
|
Table 3. Anisotropic displacement parameters(×105/ nm2)
U11 |
U22 |
U33 |
U23 |
U13 |
U12 |
|
O(1) |
51(1) |
42(1) |
42(1) |
16(1) |
-8(1) |
-3(1)
|
The planar equations of C3N3N4N5
and N1N2O1C1C2C3are:
-0.877(0.007)X+6.593(0.003)Y-4.402(0.007)Z=2.349(0.005)
-3.176(0.002)X-3.230(0.003)Y-4.384(0.006)Z=4.440(0.005)
The dihedral angel of two planes is 69.21(10) degree. Two azido groups
are not in the plane of the bis(isofurazan).
2.3 Results and discussions
The molecules of compound I and compound II are centrosymmetric. In compound II, the
bis(isofurazan) system is in a plane and the five-member heterocycle is a conjuge
structure as the furazan system. The distance of two bis(isofurazan) planes is 0.3177(2)
nm. Because two azido groups are not in the plane of bis(isofurazan) system, the density
of this energetic compound is not very high(dc=1.652 kg/m3).
The C=N bond length in bis(isofurazan) system (0.1291nm and 0.1295nm)
is between the carbon-nitrogen distances in formaldoxine (0.1276nm) and in pyridine
(0.1340nm), and it is shorter than the C=N distance in furazan (0.1300nm). The difference
suggests that there is more electron delocalization in iso-furazan than in furazan, and
there is less aromaticity in the former than in the latter.
There is no special reciprocity between two adjacent bis(isofurazn)
molecules besides the tail end nitrogen atom in the azido group and the oxygen atom of the
adjacent isofurazan ring system. The distance between these two atoms is 0.3080nm. It is
shorter than the averaging distance of two bis(isofurazan) molecule in the plane of ring
system(0.330nm).
The applications study of this azide as energetic additives are under
progress being carried out.
REFERENCES
[1] Sheremetev A B, Valentina O K, Lyndmina V B et al. Proc. Twenty-second
International Pyrotechnics Seminar, USA, July 15-19, 1996, 377.
[2] Pivina T S, Sukhachev D V, Evtushenko A V et al. Propellants, Explosives,
pyrotechnics. 1995, 20: 5.
[3] Olofson R A, Michelman J S. J. Org. Chem., 1965, 30: 1854.
[4] Saegebarth E, Cox A P. Journal of Chemical Physics, 1965, 43: 166.
[5] Vivona N, Cusmuno G, Macaiuso G. J. Chem. Soc. (Perkin I), 1977, 1616.