http://www.chemistrymag.org/cji/2001/037028pe.htm |
Jul. 1,
2001 Vol.3 No.7 P.28 Copyright |
Studies on the preparation of Fe2O3 aerogels through sol-gel process and supercritical drying technique
Gan Lihua,
Chen Longwu, Li Guangming, Yue Tianyi
(Department of Chemistry, Tongji University, Shanghai 200092, China)
Received Mar. 20, 2001; Supported by the National Natural Science Foundation of China (29973029).
Abstract Fe2O3
Aerogels were prepared from ferric trichloride and sodium hydroxide through sol-gel
process and supercritical drying technique. By means of the DTA, BET, TEM, XRD, Mössbauer
technique and so on , the aerogel samples were characterized. The results showed that
initially obtained aerogels were b- FeOOH, which would be completely converted into a-Fe2O3 through
heating treatment at 450 °C temperature. Fe2O3 aerogels, which
consist of particles with 60nm in diameters, are with uniform size and low density,
nanosized porous materials consist of nearly spherical a- Fe2O3 nano-particles. Adjusting molar ratio
of ferric trichloride and sodium hydroxide can control the density and network structure
of Fe2O3 aerogels.
Keywords Aerogels, Fe2O3, sol-gel process, supercritical
drying technique.
1. INTRODUCTION
Originally synthesized by Kistler [1] in the early thirties a variety of single
and multicomponent oxide aerogels have been prepared [2 - 7]. Aerogels are a
kind of structure under control, low density, nanosized porous materials, which consist of
nano-particles or self-coalescent high polymers, exhibiting high porosity ( 80 - 98.8% ),
surface area as 1000 m2·g-1, a
low refractive index ( 1.01-1.05 ) and sound velocity ( 100 m·s-1
), low thermal conductivity ( 0.01 W·m-1·K-1 ), and exceeding low density ( as low as 3
kg·m-3 ). Because of these unique
properties aerogels have particularly suitable for a number of application including
Cerekov detectors, insulators, acoustic impedance matching, gaseous filters, and catalytic
substrates [8 -12]. The preparation of aerogels is usually divided into two
steps [ 3, 5 ], the first step is to prepare aqueogels or alcogels by
hydrolysis and polycondensation of precursor molecules ( usually monomeric alkoxide ) in
host solvent utilizing a suitable catalyst to produce a sol-gel, the second step is to put
the gels into a high-pressure vessel to dry supercritically. Many kinds of aerogels have
been prepared through this method, however, as it is extremely difficult to prepare the
aqueogels of transition metal oxide with network structure, reports about the
preparation of one-component transition metal oxide aerogels are still rare. This paper
details synthesis and characterization of Fe2O3 one-component
aerogels. Structural aspects of the resulting aerogels have been examined using
transmission electron microscope ( TEM ), Brunauer-Emmet-Teller ( BET ),differential
thermal analysis ( DTA ), X-ray diffraction, and Mössbauer techniques.
2. EXPERIMENTAL PROCEDURES
2.1 The preparation of Fe2O3 aerogels samples
Aqueous sodium hydroxide solutions (1.50mol.dm-3) were added dropwise to
aqueous of ferric chloride (1.50mol.dm-3) with vigorous stirring, the molar
ratio of OH - / Fe3+ in the sols was
controlled by the variations of the amount of sodium hydroxide aqueous solutions, and the
composition of the aerogels is expressed in terms of the molar ratio of OH- /
Fe3+. The sols so obtained were placed into the semipermeable membrane which
was first made by collodium, then moved to the distilled water (60-65°C) for
thermoosmosis purification to remove the free Fe3+ and Cl -
in the sols until no Fe3+ and Cl- were
left in the thermoosmosis solutions. The purified sols were de-hydrated in a vacuum drier
contained phosphorus pentoxide, and homogeneous aqueogels can be formed under various
molar ratio of OH - /Fe3+. The wet gels
were immersed in pure acetone to exchange the remaining water solvent, the gels so
obtained were placed in an autoclave ( Polaron CPD ) in which the solvent was replaced in
the gels by liquid carbon dioxide ( at 4-6°C for 48h ), followed by supercritical
extraction of the carbon dioxide in critical temperature and pressure ( 32-35°C,
7.5-8.0MPa) drying unit in order to obtain the aerogel initial samples, then the Fe2O3 aerogels samples were obtained after heating
at 450°C.
2.2 Characterization techniques
Apparent density was measured from the weight and size of the aerogel samples that were
cut into common shape at first. The BET surface areas of the aerogel samples were measured
using the BET autosorb instrument (Micrometrics Flow Sorb II 2300),
the carrier gas was 30.2% N2 and 69.8% He.
DTA curve was measured using CDR-1 differential thermal analysis apparatus. Powder X - ray
diffraction patterns were obtained with a D / MAX - B X - ray diffractometer with Cuk
radiation, V=100kV, I=40mA. The crushed samples were placed into distilled water, the
shape and size of particles were observed by JEOL JEM 200 CX electron microscope whose
point resolving power is about 0.260nm. The Mössbauer spectra were obtained on samples
mounted in polyethylene cells using a constant acceleration spectrometer in the
accelerator mode at room temperature equipped with a 57Co(Rh) source. The
resultant spectra were analyzed by a constraint, least-square fit to Lorentzian-shaped
lines. The isomer shift values are quoted relative to an a-Fe absorber.
3.RESULTS AND DISCUSSION
3.1 Formation of iron polymer aquogels
During the homogeneous titration process of ferric trichloride
solutions by sodium hydroxide, the hydrolysis process of ferric chloride solutions can be
divided into four stages[ 13, 14 ]: (1) hydrolysis to mono- and dimers, (2)
reversible, rapid growth to small polymers, (3) formation of slowly reacting large
polymers, (4) precipitation of a solid phase. This process can also be described as
follows:
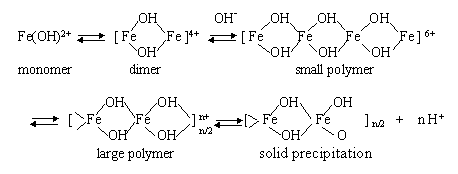
The hydrolysis process of ferric chloride solutions was controlled at the third stage in
this paper, and a hydrosol containing large amount of big polymers was formed, from which
homogeneous iron polymer aquogels could be obtained by oxolation. While adjusting the
molar ratio of OH - / Fe3+ of sols, the
network structure of the aquogels so obtained could be controlled, and this in turn
influence the structure and properties of finally produced aerogel samples.
3.2 Phase composition of initial aerogel
samples
Using the methods described in this paper, aerogel samples prepared by carbon dioxide supercritical drying of the aquogels were red brown
solids with higher strength. The phase
composition of typical samples could be described as a set of slightly asymmetric
quadrupole doublet on the Mössbauer spectrum ( Fig.1 ). The results of computer
simulation showed that the quadrupole splitting of the samples ( D= 0.68 mm/s) is quite similar to the
quadrupole splitting of b-FeOOH
as reported [ 15 ], and the as-prepared samples contain tetrahedral
framework Fe (III) with an isomer shift of 0.25mm/s[ 16 ]. b-FeOOH is one kind of substances with
anti-ferromagnetism pro-perty, whose Mössbauer spectrum using large blocks of it at the
room temperature was a typical Lexa-line spectrum with magnetic splitting. However,
when the sample particles were very small, the magnetic splitting would disappear
completely and what remained was quadrupole doublet. Meanwhile the samples appeared to be
super-paramagnetic. Consequently it was confirmed by the Mössbauer spectrum that the
initial samples were aerogels with network structure composed of ultrafine b- FeOOH particles. This
conclusion is also verified by the measurement results of XRD.
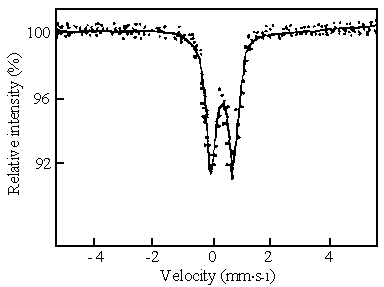
Fig.1 Mössbauer spectrum of initial aerogel samples
prepared from the 1.50 molar ratio of OH- / Fe3+ recorded at room
temperature.
3.3 Phase transition of aerogels
The initial samples were put into the differential thermal analysis apparatus and the
temperature increased according to a set program to obtain the DTA curve ( Fig.2 ). The
endothermic peak occurred at 74°C is a desorption peak of adsorbed water from the
surfaces. Because the samples were porous with relatively large specific surfaces, the
adsorption on them was especially strong and the desorption peak of adsorbed water was
quite broad. The other endothermic peak occurred at 170°C was caused by b-FeOOH losing of structural water when
heated and converted to a-Fe2O3.
The exothermic peak, at 285°C, was a phase transition peak caused by the conversion from a- Fe2O3 to poorly
crystallized a- Fe2O3
, while the endothermic peak at 406°C whose form was very regular and sharp was the
crystallization peak of the poorly crystallized a-Fe2O3[17]. So the
process of phase transition of aerogel samples could be described as follow:
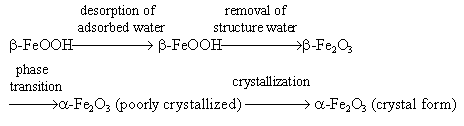
The DTA study showed that the phase transition to a-Fe2O3 had been
completed after heating to 406°C.
The results of XRD measurements of initial and after-heating samples confirmed the
above-mentioned phase transition.
The position of XRD characteristic peaks of the initial aerogel samples prepared from
solutions with different molar ratio of OH- / Fe3+ were almost the
same, and the XRD spectrum of typical samples is showed in Fig.3 ( a ). The composition of
the samples could be confirmed to be b-FeOOH in accordance with the characteristic peak values of its
peak acme (7.55, 5.38, 3.32, 2.54, 2.30, 2.08, 1.95, 1.74, 1.64, 1.51, 1.44, 1.37 ), and
the disperse peak lines appearing on the XRD spectrum was evident
that the particles of samples were extremely small. Fig.3( b) shows the XRD spectrum of
aerogel samples after heating at 450°C, it can be seen that the aerogel samples prepared
in this method are crystal form a-Fe2O3 from their characteristic peak value
of the XRD pattern (3.68, 2.70, 2.51, 2.20, 2.08, 1.84, 1.70, 1.60, 1.48, 1.45 ).This
Conclusion is still supported by the research of Mössbauer spectrum
[ 18 ].
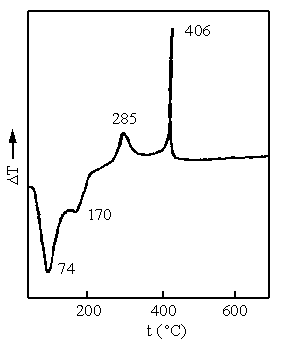
Fig.2 DTA curve of an aerogel prepared from the 1.50 molar ratio of OH-/Fe3+
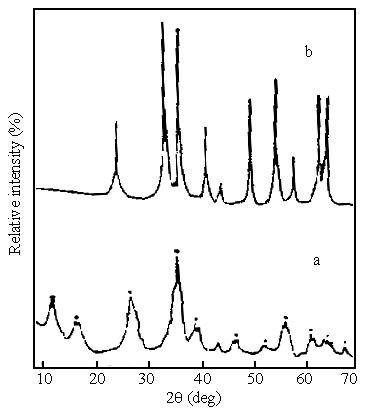
Fig.3 XRD pattern of aerogel samples prepared from the 1.50 molar ratio of OH-/Fe3+
(a) initial samples after supercritical drying (b) samples after heating at 450°C
3.4 Structure of typical iron oxide aerogel samples
Typical a-Fe2O3
aerogel samples are brown porous solids with higher strength, whose transmission electron
micrograph ( TEM ) is shown in Fig.4. These aerogel samples were produced at a solution:
molar ratio of OH- / Fe 3 + = 1.50. Statistical analysis of particle
size seen in the TEM, shows that the particle size of the aerogel samples was 60nm in
diameter. The particles were nearly spherical and the size distribution was quite narrow.
This result indicated that the aerogels prepared by this method were a kind of narrowly
distribution, low-density, nanosized porous solid materials consisted of nearly spherical a-Fe2O3
nano-particles.
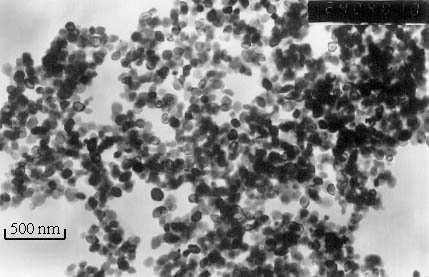
Fig.4 Transmission electron micrograph of an aerogel derived from the 1.5 molar
ratio of OH-/Fe3+
3.5 The effects of the preparing conditions
on properties of aerogels
Fe2O3 aerogel samples produced by the same method with different
molar ratio of OH-/Fe3+ showed that the primary particles are
similar, but the density and the specific surface area of the
aerogel samples changed. The measured density and specific surface area of the
aerogel samples with OH-/Fe3+molar ratio varying from 0.40 to 2.00
are shown as table 1. With increasing molar ratio of OH-/Fe3+, the
density of the aerogels increased, and the specific surface area of the aerogels
decreased. These observations can be interpreted by considering that shrinkage in our
system should occur from condensation between adjacent groups during the long aging and
solvent exchange times following thermal dialysis. Since the reaction medium ( water ) is
the most probable source of oxo ligands required for condensation, the increase in
hydroxide ion ( i. e. increased pH ) increases the potential for deprotonation of ligand
water and hydroxo groups leading to a higher degree of oxolation. These will cause the
solutions with relatively large molar ratio of OH- / Fe3+ to produce
finally aerogels with large degree of cross-linking and dense network, so their density
was large and specific surface was small.
Table 1 The result of the density and specific surface area of the aerogel samples
| Samples | molar ratio of OH-/Fe3+ |
||||
0.40 |
0.60 |
1.00 |
1.50 |
2.00 |
|
| Density/kg.m-3 | 240 |
261 |
310 |
410 |
475 |
| Specific surface area/m2.g-1 | 342.2 |
276.4 |
244.4 |
224.0 |
193.9 |
4. CONCLUSIONS
1. Fe2O3 aerogels have been prepared, as low-density porous solid
materials, with network structure that consists of spherical nano-particles with sizes of
about 60 nm.
2. Adjusting the molar ratio of OH- / Fe3+ can control the network
structure of Fe2O3 aerogels. The present study shows that the
increase of molar ratio of OH- / Fe3+ will make the network
structure of aerogels become dense, whence the increase of density.
3. According to the preparing method in this paper, the initial
samples are a porous network materials
consisted of b-FeOOH
ultrafine particles.
REFERENCE
[1] Kistler S S. J. Phys. Chem., 1932, 36: 52.
[2] Gesser H D, Goswami P C. Chem. Rev., 1989, 89: 765.
[3] Fricke J, Emmerling A. Struct. Bonding(Berlin) (Spectrosc. Appl. Sol-Gel Glass) ,
1992, 77: 89.
[4] Chen L W, Gan L H, Yue T Y et al. Chem. J. Chinese Univ. (Gaodeng Xuexiao Huaxue
Xuebao),1995, 16: 840.
[5] Chen L W, GAN L H. Chemistry (Huaxue Tongbao), 1997, (7): 21.
[6] Fricke J, Emmerling A. J. Sol-Gel Sci. Tech., 1998, 13: 299.
[7] Guo Yizhu, Guadalupe A R. Chem. Commun., 1999, (4): 315.
[8] Rubin M, Lampert C M. Solar Energy Mater., 1983, 7: 393.
[9] Gronauer M, Fricke J. Acustica, 1989, 59: 177.
[10] Cooper D W. Part. Sci. Technol., 1989, 7: 731.
[11] Hrubesh L W, Poco J F. Mater. Soc. Symp. Proc. (Adv. in Porous Mater.), 1995, 377:
195.
[12] Gougas A K, Ilie D, Ilie S et al. Nucl. Instrum. Methods Phys. Res., Sect. A, 1999,
421(1,2): 249.
[13] O'Sullivan E C, Ward A J I, Budd T. Langmuir, 1994, 10: 2985.
[14] Gan L H, Yue T Y,. Li G M et al. Acta Physico-chimica Sinica. 1997, 13(1): 48-51.
[15] Johnston J H, Logan E. J. Chem. Soc. Dalton Trans., 1979, (1): 13.
[16] Meagher A, Nair V, Szostak R. Zeolites, 1988, 8: 3.
[17] Paterson E, Swaffield R, Clark D R. Thermochimica Acta , 1982, 54: 201.
[18] Gan L H, Li G M, Yue T Y et al. Chem. J. Chinese Univ. (Gaodeng Xuexiao Huaxue
Xuebao),1999, 20(1): 132.