http://www.chemistrymag.org/cji/2001/037029pe.htm |
Jul. 1,
2001 Vol.3 No.7 P.29 Copyright |
Xu
Shukun, Zhou Huanying, Du Xiaoguang, Zhu Daan
(Research Center for Analytical Sciences, Box 332, Northeastern University, Shenyang,
110006, China)
Received Jan.3, 2001; Supported by Natural Science Foundations of China(No. 29975005).
Abstract
Volatile species of Cu, Au and Tl were generated at room temperature by the reduction of NaBH4 using a flow injection vapor generation atomic absorption spectrometric (FI-VGAAS) system. The 1, 10-phenanthroline, sodium diethanlydithio-carbonate (DDTC) and palladium (or gold, platinum) were used as enhancement reagents respectively. Palladium combined with Rodamine B showed co-enhancement effect on gold. The normal flow injection peak shape was obtained and the sensitivities were enhanced by 1 - 2 magnitude. The detection limits of 0.34 - 24 ng/ml (3s ) were obtained with 300 or 500 ml sample solution. The precision of 2.6 - 1.8% RSD were obtained with the sampling frequencies were of 120 -180/h. The FI-VGAAS methods were used for the determination of copper in hair and rice sample and gold in the ore sample digests.Keywords chemical vapor generation AAS, flow injection, copper, gold, thallium, enhancement reagent.
1 INTRODUCTION
Flow injection vapor generation atomic absorption spectrometry (FI-VGAAS) has been proved
to offer significant advantages, including more than 90% reduction in sample and reagent
consumption, 2-3 fold higher sampling frequencies, better precision, enhanced selectivity
and automated operation. Because of these benefits, the FI-VGAAS technique has been
extended to all chemical vapor formation elements such as Se, Te, As, Sb, Bi, Pb, Ge, Sn,
Hg etc using quartz tube atomizer or graphite furnace atomizer with in situ trapping
preconcentration [1]. But FI-VGAAS of copper, gold have not been reported up to
now, reports on the VGAAS of thallium are very few either, and the sensitivity was limited
by low vapor generation efficiency. Some preliminary research works on this field for
searching the enhancement reagents were undergoing in the author's group, and some
progresses were achieved. Using FI-VGAAS system, it was found that the volatile specie of
copper, gold and thallium may be generated in the presence of enhancement reagent. Even
though it was proved by our experiments that both cadmium and silver in acidified aqueous
medium could generate volatile species by reduction with NaBH4 without any
enhancement reagent [2,3], the volatile species of copper, gold and thallium
can not be generated in the FI system without enhancement reagent. Some new reports on the
vapor specie generation of the three elements using a non- FI model gave very low
sensitivity [4,5]. In our recent studies, we found that the vapor specie of
copper could be generated in the presence of micro amounts of 1,10-phenanthroline; the
volatile specie of gold could be generated in the presence of micro amounts of sodium
diethyldithiocarbamate (DDTC); the hydride vapor of thallium could be generated with an
enhanced sensitivity in the presence of micro amounts of palladium (or gold and platinum).
The preliminary FI-VGAAS determination methods were developed for Cu, Au and Tl
respectively [6-8].
2 EXPERIMENTAL
2.1 Apparatus
A Perkin-Elmer Model 2100 atomic absorption spectrometer with a deuterium lamp background
corrector was used combined with a Model FIAS-200 flow injection system and the attached
mercury and hydride generation system. Copper, gold and thallium hollow cathode lamps were
operated at 10, 15 and 10 mA respectively. Wavelength of 324.7, 242.8 and 276.8nm were
used respectively with a band pass of 0.7, 2.0 and 0.7 nm. The peak height absorbance was
used for evaluating the results throughout the work. The time-resolved absorbance signals
for the interest elements were recorded by high-resolution graphics on a high-resolution
screen and printed out using an Epson EX-800 printer (Epson Japan). The rotations of the
two multi-channel peristaltic pumps and the actuation of the valve were programmed and
automatically controlled by the computer software. The gas-liquid separator (GLS, Zhao-Fa
Automatic Analysis Institute, Shenyang) used is similar to the transparent plastic
separator with one third filled glass beads of 3 mm diameter described by Fang 1.
High-purity argon was used as carrier gas.
2.2 Reagents and Standard Solution
Analytical grade reagents and de-ionized water were used throughout. A 0.3 -1% (m v-1)
solution of sodium tetrahydroborate was prepared daily in de-ionized water containing
sodium hydroxide (0.3%, m v-1) to keep the reductant stable.
1,10-phenanthroline (0.4%, m v-1, Shenyang Chemical Co.
Shenyang, China) was prepared by dissolving 0.2 g of 1,10-phenanthroline in 50ml deionized
water.
Nitric acid (63%, w v-1, Jinxi Chemical Co., Jinxi, China)
was reagent grade.
Hydrochloric acid (36%, w v-1, Jinxi Chemical Co., Jinxi,
China).
The working stock solution of 10mg L-1 of Cu, Au and Tl was
prepared by dilution of the stock standard solutions (1g L-1). A series of
working standard solution were prepared by stepwise dilution of the 10 mg L-1
working stock solution and made to contain the needed acidity and enhancement reagent
concentration for the interest elements.
2.3 Procedure
The FI manifold used for VGAAS is shown in Fig. 1 together with the optimized operating
parameters listed in Table 1 for the interest elements. As shown in Fig.1, in loading
step, standard or sample solution was filled into the sample loop. In injection step,
ending the sampling, the valve rotated automatically to injection position. Pump 2 was
speeded up (as in Table 1), and the sample or standard solution stored in the sampling
loop was carried out by carrier and merged with reductant. The chemical reaction took
place immediately to form volatile specie and the vapor was separated from the waste in
the gas-liquid separator (SP) meanwhile the generated vapor was carried by argon carrier
into the quartz tube atomizer (AAS) at 1000°C for detection.
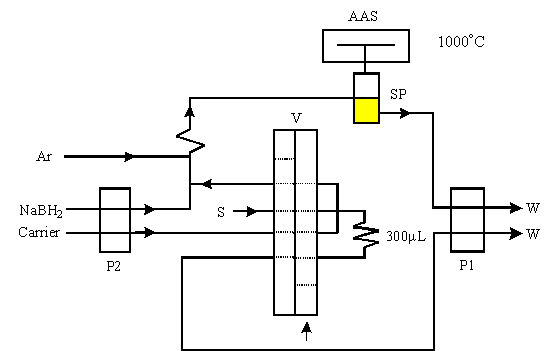
Table 1 Flow injection operation program for the vapor generation
| Step | Valve |
Element |
Time (S) |
PumP 1 (ml/min) |
PumP 2 (ml/min) |
Function |
1 |
Fill |
Tl |
4 |
S:3.2, W:25 |
R:2.0, C:6.0 R:2.6, C:5.6 |
Prefill and change sample |
2 |
Fill |
Tl |
12 |
S:4.4, W:25 |
R:1.2, C:3.6 R:2.6, C:5.6 |
Sample filling |
3 |
Injection |
Tl |
12 |
W:25 |
R:3.0, C:9.2 |
Sample injection |
3 RESULTS AND DISCUSSION
3.1 Effect of atomizer temperature
The preliminary experimental results showed that the temperature of
quartz tube atomizer had great effects on the detection signal. The experimental results
on the relationship between the peak height absorbance and the atomizer temperature showed
that higher temperature can provide enough energy to atomize the volatile copper, silver
and gold, which lead to a almost linear increase from 700°C to 1000°C.
Because of the software limitation (the maximum temperature is not allowed beyond 1000°C),
999°C was used for atomizing in further experiments.
3.2 Selection of enhancement reagent
For the generation of copper volatile specie, steurgen et al [9] reported a
continuous flow system with ICP-AES detection. But with FI- AAS detection, the sensitivity
is too low to be detected at ng ml-1 levels [6]. DDTC, EDTA,
thiourea, ethanol and 1.10-phenanthroline were tested for enhancing the reaction
sensitivity. Only micro amounts of 1.10-phenanthroline showed a good enhancement role.
According to the experimental results, 0.00048% of 1.10-phenanthroline in sample solution
was used in the further experiments. The sensitivity was enhanced by a factor of 15.
For the hydride generation of thallium, metal elements such as Au, Pt,
Pd, Ni, Co and dyes such as rohdamune B, chromium-black T, methyl purple were tested for
the enhancement reagent. It was found that Au, Pt and Pd elements in the sample solution
at a certain concentration could enhance the signal of thallium at mg L-1 level. Especially
palladium showed an excellent performance for enhancing the hydride generation efficiency
of thallium among the three metal ions and it is the cheapest one, so it was chosen as the
enhancement reagent. According to the optimization results, 400ng ml-1 of Pd in
sample solution was used in the further experiments. Compared to the data from reference [4]
without and with the presence of tellurium, the sensitivity was increased by a
factor of 220 and 28 respectively. Compared with the signal of 10mg L-1
thallium obtained using this FI system without the presence of palladium and with the
presence of 4mg L-1 tellurium, the sensitivity was increased by a factor of 40.
Experimental results also showed that Rhodamine B has a co-effect of enhancing the peak
height of thallium in the presence of palladium. The peak height absorbance of thallium
was enhanced by further 40% with the presence of 0.0005% of Rhodamine B in the sample
solution under the same FI parameter and reagent concentration, except that the optimum
nitric acid concentration in sample solution was reduced from 0.2 mol L-1 to
0.1mol L-1.
For the VGAAS determination of gold, it is more important to choose an
appropriate enhancement reagent for enhancing the vapor generation efficiency of gold
using quartz tube as atomizer, because the sensitivity of the VGAAS system without
enhancement reagent is too low as mentioned in reference 5. 1,10 (o)-phenanthroline,
8-hydroxyquinoline, Rhodamine B, 1-pyrrolidine dithiocarboxylic acid ammonium salt (APDC)
and DDTC was tested for the enhancement reagent selection respectively. It was found that
only micro amounts of DDTC in the sample solution showed marked enhancing effect on the
signal of gold. According to experimental results, 0.03% DDTC in sample solution was
chosen as the optimum concentration. The signal peak height absorbance was enhanced by a
factor of 21 compared with the signals obtained without using enhancement reagent and a
factor of 48 (for 10 ml
sample) or 96 (for 20 ml
sample) compared with reference 5.
3.3 Effects of FI and chemical parameters
3.3.1 Acidity of sample and carrier solution
Generation efficiency of thallium hydride depends on acidity of reaction medium and the
acid species strongly. An attempt was made to investigate the effects of HCl, HNO3
and H2SO4 acidity from 0.05mol L-1 to 2mol L-1.
Experimental results showed that low-acidity conditions were benefit for producing higher
sensitivity for thallium hydride generation. But when hydrochloric acid was used, the
signal was unstable, and the significant decrease in peak height was observed when the
concentration of hydrochloric acid up to or beyond 0.3 mol L-1. The reduction
in sensitivity with high chloride ions was at least partially due to the formation of
Tl-Cl complex, which quench the reaction of thallium ion with sodium tetrahydroborate for
forming thallium hydride. For H2SO4 medium, SO42-
can form yellowish precipitation with thallium ion, so may not be the best choice either.
Using nitric acid as sample medium, a plateau was existed in the nitric acid concentration
from 0.1mol L-1 to 0.3 mol L-1. The high nitric acid concentration
has negative effect for the determination due to the dilution effect of large account of
hydrogen generated in the reaction process.
From the results on the effect of sample solution's acidity on the peak
height absorbance of 100 ng ml-1 copper and 2.0 mg l-1 gold, the
optimum 0.1mol/L nitric acid and 0.1mol/L hydrochloric acid were chosen as sample solution
acidity respectively.
3.3.2 Effects of sodium tetrahydroborate concentration
According to the optimization results about the effects of NaBH4 (in 0.3% NaOH)
concentration on the signals of copper, gold and thallium with the presence of enhancement
reagent, 0.3%, 1.0% and 0.8% of NaBH4 in 0.3% NaOH solution were chosen as
optimum concentration respectively for thallium, copper and gold respectively.
3.3.3 Effects of reaction coil length and sample volume
The effects of reaction coil length on the peak height absorbance were investigated in the
range of 4 - 16 cm. The shortest length of 4 cm corresponded to the length connecting the
reagent merging point and gas-liquid separator. Because the vapor generation reaction is
an instant quick reaction, and shorter reaction times are beneficial for suppressing
interfering reaction slower than the main reaction, so longer reaction coil has a negative
effect on determination. The reaction length of about 10cm was used for high sensitivity.
There was not evident difference in sensitivity when the sample volume
beyond 500ml for copper and
thallium. As for gold, there is not marked increase in the signal peak height absorbance
for the sample volume beyond 300 ml. So 500ml for copper and thallium as well as 300 ml for gold were chosen as the optimum sample volumes respectively.
3.3.4 Flow rate for sample, reagent and carrier gas
The effects of carrier and reagent flow rates on the peak height absorbance were
investigated while keeping the ratio of carrier/reagent flow rate as a appropriate ratio
value, i.e. selecting a matched well pump tubes for carrier and reagent. Then optimization
experiments on the relationships between peak height absorbance and carrier flow rates
were made for the three elements. As a result, the carrier flow rate of 9.2 ml min-1
for thallium and copper, 7.0 ml min-1 for gold were used, with their reagent
flow rate of 3.0, 4.6 and 4.0 ml min-1 respectively.
In the three FI-VGAAS systems, argon was used as carrier gas to
transport the vapor specie into atomizer. The flow rate and flow stability of carrier gas
usually has significant effect on the sensitivity and repeatability of the method.
Experimental results showed that 140, 200 and 400 ml min-1 of argon flow rate
were the optimum carrier gas flow rate for copper, thallium and gold respectively.
3.4 Interferences
Cd, Hg, Cu and Pb interference the process of thallium hydride generation. It was tried to
reduce or eliminate the interferences by increasing acidity of sample solution or using a
masking reagent. It was tried to reduce or eliminate the interferences by increasing
acidity of sample solution or using a masking reagent. When the sample acidity was raised
up to 1mol L-1, the interference from cadmium and mercury ions were almost
eliminated but the sensitivity was lost by 39% and 20% respectively. When 0.002%
1,10-Phenanthroline was added in sample solution as masking reagent, the interference from
Cd, Hg and Cu can be eliminated basically, making the tolerant concentrations of Cd, Hg
and Cu up to 1.0, 0.5 and 0.5 ml L-1 respectively. The interference from lead
was not easy to be eliminated by using the masking reagent due to its characteristic
similar to thallium. Using the self-making column packed with sulfydryl resin and keeping
the sample at the rate of about 14ml min-1 flow through the column can
eliminate the interference from lead.
For the determination of copper, nickel at equal amounts of copper in
sample solution showed -13% interference on the detection signal.
For the determination of gold, 20 kinds of possible co-existing metal
ions were tested for the interference study with and without the presence of EDTA in
sample solution. The experimental results showed that when 400 - 800 mg L-1
EDTA was added in sample solution as masking reagent, the interference from Zn(II),
Fe(III), Co(II), Ni(II), Pb(II), Cd(II), Cr(III), Ge(IV), As(III) and Al(III) can be
eliminated basically. The interferences from Cu and Ag were not easy to be eliminated by
the masking reagent due to their characteristic similar to Au.
3.5 Analytical Performance
With the optimum conditions of the FI-VGAAS systems, the characteristic data for the
typical analytical performances of the three elements are listed in Table 2.
River water, tap water and mineral water sample were analyzed using the
method. The thallium in these water samples was not detectable for the levels were too low
to be determined. The recoveries by spiking 100 mg L-1 thallium were of 101-109%. The recoveries of gold
in reservoir water, tap water and seal water sample were measured. The gold in these water
samples was not detectable either for the levels were also too low to be determined.
Table2 Characteristic performance data of FI-AAS
| Element | Enhancement reagent |
DL
(3s ) |
RSD |
Enhancement factor |
Linear
range |
Tl |
Palladium |
6.7 |
1.2 |
220a, 40b |
0 - 250 |
Palladium * |
3.4 |
0.9 |
308a, 56b |
0 -400 |
|
Cu |
1,10-penanthroline |
1.8 |
2.6 |
10 |
0 - 150 |
Gold |
DDTC |
24 |
2.0 |
21 |
0 - 2000 |
a
Compared with reference 4, without the presence of tellurium;b Compared with the signal of 10 mg/l Tl of obtained with the presence of 4 mg/l of tellurium;
* With the presence of 0.0005% (m v-1) Rhodamine B.
The recoveries by spiking 1.0 mg L-1 gold in the samples were 99%, 101% and 91% respectively. The method was applied to the determination of gold in ore sample after active carbon separation and preconcentration, the results were in good agreement with those obtained by flame AAS. The FI-VGAAS method for the determination of copper was used for the determination of copper in hair and rice SRM sample, the results were in good agreement with the certified values.
4 CONCLUSION
The results indicated that efficient enhancement reagent are useful for the chemical vapor
generation reaction. Using a quartz tube atomizer at room temperature with a atomizer
temperature of 1000°C, micro amounts of palladium (or Pt, Au) in sample
solution is an efficient enhancement reagent for thallium hydride generation. Micro amount
of DDTC in sample solution is an efficient enhancement reagent for the generation of
volatile specie of gold. The vapor specie of copper can be generated in the presence of
micro amounts of 1,10-phenanthroline. Study on the mechanism of the enhancement effects is
undergoing.
ACKNOWLEDGMENT
The authors wish express their thanks to Professor Zhaolun Fang for his helpful
discussions. The financial support from Natural Science Foundations of China, and
Bodenseewerk Perkin-Elmer GmbH, Germany, for providing the atomic absorption spectrometer
and their partial financial support, are also gratefully acknowledged.
REFERENCES
[1] Fang Z L. Flow Injection Atomic Absorption Spectrometry, Chichester, John Wiley and
Sons, 1995.
[2] Liu MY, Xu SK. At.Spectrosc., 1997, 18.(6): 195.
[3] Xu S K, Liu M Y, Xu Z R et al. Rock and Mineral Analysis (Yan Kuang Ce Shi), 1999, 18:
15.
[4] Yan D, Yan Z, Cheng G et al. Talanta, 1984, 31: 133.
[5] Luna A S, Sturgeon R E, Campos RC. Anal. Chem., 2000, 72, 3523.
[6] Zhou H Y, Xu S K, Fang Z L. Spectroscopy and Spectral Analysis (Guangpuxue yu Guangpu
Fenxi), 2000, 20: 528.
[7] Du X G, Xu S K, Fresenius. J. Anal Chem, Accepted.
[8] Zhu D A, Xu S K. Spectrosc, 2000, 21: 136.
[9] Sturgeon R E, Liu J, Boyko V J et al. Anal Chem. 1996, 68.(11): 1883.