http://www.chemistrymag.org/cji/2001/037030ne.htm |
Jul. 1,
2001 Vol.3 No.7 P.30 Copyright |
New isocoumarins from the mangrove endophytic fungus #2533
Lin Yongcheng, Wan Jun, Zhou Shining, Gareth Jones E B#(Department of Appied Chemistry, Zhongshan University, Guangzhou 510275; #Department of Biology and Chemistry, City University of Hong Kong, China) Received Feb.16, 2001. Supported by the National Natural Science Foundation of China (29672053, 20072058), the Natural Science Foundation of Guangdong Province, China (950026 and 980317) and a strategic grant at City University of Hong Kong (7000650).
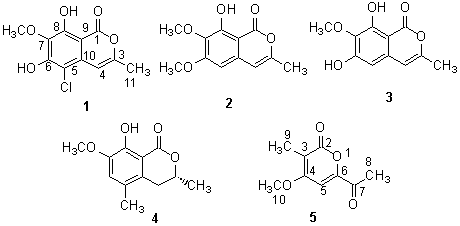
Abstract Two new isocoumarins avicennin A(1) and B (4) with two analogues(2, 3) and vermopyrone(5) were isolated from the mangrove endophytic fungus (strain #2533) from the South China Sea. Their structures were elucidated using spectroscopic methods, primarily 2D NMR techniques.
Keyword mangrove endophytic fungus, isocoumarins, metabolites. Endophytes are all organisms inhabiting plant materials that some time in their life, and can colonise internal plant tissues without causing apparent harm to their host [1]. Endophytic fungi are normally restricted to healthy plant parts where symptoms of fungal infection cann't be detected. It was hypothesized that the endophyte-host interaction was a balanced antagonism probably involving secondary metabolites produced by the partners [2] Endophytic fungi are now recognized as potential producers of novel secondary metabolites, which can be used as possible biocontrol agents and drugs .[3] Although a variety of new compounds have been discovered from fungi isolated from the marine environment recently,[4 - 8] there is no records of metabolites or novel compounds from endophytic fungi of mangrove plants to date. We have first isolated and characterized such fungal metabolites from leaves of the mangrove plants from the coasts of South China Sea [9]. In this paper, we report two new compounds, avicennin-A (1) and B (4) and two analogues (2) and (3), and (5) obtained from an endophytic fungus isolated from young leaves of the mangrove plant Avicennia marina in the Pearl River Estuary of Southern China.
.
1. RESULTS AND DISCUSSTION
A 220L fermentation broth was concentrated and extracted with ethyl acetate. The extract
was repeatedly chromatographed on silica gel columns. avicennin-A (1) was
obtained as colorless needles, mp. 197-199oC. 1 has the molecular
formula C11H9ClO5 as determined by FABMS and elemental
analysis. The isotopic peak of molecular ion showed that 1 contained chlorine atom
and this was confirmed by X-ray photoelectron spectroscopy. The numbers of hydrogen and
carbon atoms in the 1H and 13C NMR spectra were in agreement with
the molecular formula.
The IR spectrum of 1 showed the presence of carbonyl (s) and
hydroxyl group (s) with bands at 1690 cm-1and at 3436 cm-1.
The 13C NMRspectra of 1 exhibited eleven carbon signals,
except two methyl and a carbonyl group, the other eight signals were all unsaturated
carbons (equivalent to four double bonds). The seven unsaturation equivalents required by
the molecular formula indicated the compound has two rings. In the 1HNMR
spectrum, the hydrogen-bond hydroxyl proton at d11.37 showed that it was adjacent to the carbonyl group. These
implied that 1 was a substituted isocoumarin. The HMBC spectrum confirmed the
deduction (see Fig.1). Although no any correlation signal orienting of the hydroxyl goup
(at
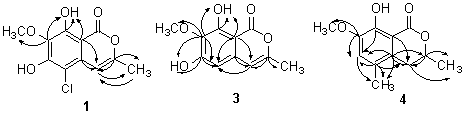
Fig.1 The HMBC correlations of 1,3 and 4
2 and 3
were analogues of 1 and had identical 1H, 13CNMR,
MS and melting point data to that published for two isocoumarines from Streptomyces
mobaraensis[11].
The steric conformation of the lactone ring in this formulation was deduced from the diaxial relationship of protons at positions 3 and 4 (JBX=12Hz) found in the 1HNMR of 4. Hence, the structure of avicennin B was elucidated to be 3,4-hydro-8-hydroxy-7-methoxy-3a,5-dimethyl -isocoumarin (4).
The structure of 5 was elucidated by its spectra. It is the known compound, vermopyrone, a metabolite of Gliocladium vermoesenii. The biosynthesis study of vermopyrone showed that it was consistent with a polykitide origin with extra methyl groups introduced from methionine. [12-13] Although it has been well-known that 1-4 were also derived biosynthetically from an acetate unit, it seems that there was biosynthetically no direct relationship between 5 and 1-4.
It is probably a feature that isocoumarins, the chlorine-containing isocoumarin and the dihydroisocoumarin coexisted in this endophytic fungus. Anderson and coworkers's systematic survey of the Xylariaceous fungi led to isolation of seven dihydroisocoumarins and other metabolites, but nor any isocoumarins was found from those fungi.[11] On the other hand, Eaton et al found four isocoumarins without dihydroisocoumarins from Streptomyces mobaraensis.[12] As our knowledge, it has not been reported that isocoumarins, chlorine-containing isocoumarin and dihydroisocoumarins were simultaneously isolated from a fungus.
Table 1. NMR data of 1 and 4
1 |
4 |
|||||
13 C |
1 H |
HMBC |
13 C |
1 H |
HMBC |
|
| 1 | 166.2 (C) |
170.6 (C) |
||||
2 |
||||||
3 |
154.5(C) |
H-4, 11 |
76.0(CH) |
4.67(qdd,6.5,3,12Hz) |
H-4, 11 |
|
4 |
100.8(CH) |
6.74(d, 1Hz) |
H-11 |
31.4(CH2) |
a.2.99 (dd, 3, 17Hz), |
H-11 |
5 |
131.6(C) |
127.5 (C) |
H-4, 6, 12 |
|||
6 |
152.7* (C) |
119.7 (CH) |
6.89 (s) |
H-12 |
||
7 |
133.2(C) |
H-12,OH-8 |
146.7 (C) |
H-6, 13 |
||
8 |
152.8*(C) |
OH-8 |
150.7 (C) |
H-6 |
||
9 |
100.2(C) |
H-4, OH-8 |
124.3 (C) |
H-3,4, 6, 12 |
||
10 |
104.8(C) |
H-4 |
108.2 (C) |
H-4 |
||
11 |
19.7(CH3) |
2.31 (d, 1Hz) |
H-4 |
18.4 (CH3) |
1.54 (d, 6.5Hz) |
|
12 |
61.1(CH3) |
4.06 (s) |
20.9 (CH3) |
2.21 (s) |
||
13 |
56.3 (CH3) |
3.89(s) |
||||
OH-8 |
11.37 (s) |
OH-8 |
11.20 (s) |
|||
OH-6 |
6.60 (s) |
|||||
*unassigned
2. EXPERIMENTAL
2. 1 General
NMR data were recorded on a Varian Inova 500NB NMR spectrometer, mass spectra on a
VG-ZAB-HS mass spectrometer, IR spectra on Bruker EQUINOX 55 spectrophotometer, UV spectra
on a Shimadzu UV-2501PC spectrophotometer, optical rotations on a Horiba High Sensitivity
Polarimeter SEPA-300, elemental analyses on a Elementar Vario EL CHNS-O elemental analyzer
and on a S-520 scan electronic microscope/Link ISIS-300 X-ray photoelectron spectroscope
(SEM/EDS).
2. 2 Fungal strain
A strain of the fungus (#2533), an endophytic fungus, was isolated from mangrove young
avicennia leaf from the South China Sea Coast, and was stored in Department of Applied
Chemistry, Zhongshan University Guangzhou, P. R. China.
2. 3 Culture conditions
Starter cultures (from Professor E. B. G. Jones and Dr. L. L. P. Vrijmoed) were
maintained on cornmeal seawater agar. Plugs of agar supporting mycelial growth were cut
and transferred aseptically to a 250mL Erlenmeyer flask containing 100mL liquid medium
(glucose 5 g/L, peptone 1g/L, yeast extract 0.5 g/L, beef extract 0.5g/L, natural sea
water 50ml/L). The flask was incubated at 30oC on a rotary shaker for 5-7 days.
The mycelium was aseptically transferred to 500ml Erlenmeyer flasks containing culture
liquid (200mL). The flasks were incubated at 30oC for 45 days.
2. 4 Extraction and separation of metabolites
The cultures (220 L) were filtered through cheesecloth. The filtrate was concentrated
to 5 L below 50oC, and extracted three times by shaking with an equal volume of
ethyl acetate. The combined extracts were chromatographed on silica gel using a gradient
elution from petroleum to ethyl acetate to obtain3 (55mg), 2 (20mg), 5(60mg),
4 (50mg) and 1(120 mg) in turn from the 10-20% ethyl acetate/petroleum ether
fraction.
1 colorless needles: mp. 197-199oC. IR (KBr) 3436, 3010, 2955, 2850,
1690, 1646, 1560, 1472, 1345, 1229, 1174, 1095, 795 cm-1; UV:lmax(CHCl3) 247 nm
(e51571), 359nm (
2 colorless needles, mp198-200oC. FBMS 237(M+1). 1H NMR (CDCl3,TMS),d2.26(CH3 ), 3.94(CH3 ), 3.90 (CH3 ), 6.33(s, 1H), 6.18(s, 1H), 11.06(s, 1H). 13C NMR (CDCl3) d166.4(C),160.0(C),154.7(C),154.3(C), 153.3 (C), 135.1 (C), 134.5(C), 104.8(C), 98.0(CH), 60.8(CH3), 56.1(CH3), 19.36(CH3) (lit.,[10] mp199oC).
3 colorless needles, mp194-196oC, FBMS 223(M+1). 1H NMR (CDCl3,TMS),d2.25(CH3-11 ), 4.03(CH3-12 ), 6.15(s, H-4), 6.39 (s, H-5), 6.46(s, OH-6), 11.31(s, OH-8). 13C NMR (CDCl3)d166.6(C-1), 156.3(C-6), 153.7 (C-8), 153.2(C-3), 134.5(C-5), 132.6 (C-7), 104.2(CH-4), 101.2 (C-10), 100.3(C-9), 60.9 (CH3-12), 19.3(CH3-11). the HMBC correlations see Fig.1 (lit.[10] mp 194 oC).
4 colorless plates, mp 159-160oC, [a]D22 = -53.8º. EIMS M/z 222(M)+, 207, 204, 193, 189, 176. IR (KBr) 3328, 3266, 3050, 3014, 2981, 2894, 1663, 1596, 1476, 1385, 1262, 1110, 740 cm-1. UV: lmax (CHCl3) 256 nm (e5471), 340nm (e4502). 1H NMR (CDCl3, TMS), 13C NMR (CDCl3) and 2D NMR see Table 1. Anal. Found: C65.23, H 6.62, Calcd.(for C12H14 O4): C 64.87, H 6.31.
5 colorless needles, mp 138-140oC, FBMS 183(M+1). 1H NMR (CDCl3,TMS),d2.01(CH3 ), 2.55(CH3 ), 3.96 (CH3 ), 7.07(s, 1H). 13C NMR (CDCl3) d191.6(C), 164.0 (C), 163.2(C),153.1(C), 109.7 (C), 98.0(CH), 56.7(CH3), 25.8(CH3), 9.4(CH3) (lit.,[11] mp 139-141).
ACKNOWLEDGMENTS
Spectra were taken in the Instruments Analytic Center of Zhongshan University. E.B.G. Jones acknowledges the Royal Society, U.K. and City University of Hong Kong for the award of the Kan Tong Po Visiting Professorship.
REFERENCES
[1] Petrini O. Fungal endophytes of tree leaves. In: Microbial ecology of leaves.
(eds. Andrews J H & Hirano SS), New York: Springer-Verlag,1991, 179.
[2] Schulz B, Rommert A K, Dammann U et al. Mycological Research, 1999, 103: 1275.
[3] Rodrigues K F, Petrini O. Biodiversity of endophytic fungi in tropical regions. In:
Bio diver- sity of Tropical Microfungi (ed. K D Hyde), Hong Kong: Hong Kong University
Press, 1997, 57-69.
[4] Lin Y, Shao Z, Jiang G et al. Tetrahedron, 2000, 56 (49): 9607.
[5] Lin Y, Wu X, Fen S et al. Tetrahedron Letters, 2001, 42: 449.
[6] Belofsky S, Jensen G N, Fenical W et al. Tetrahedron Letters, 1999, 40 (15): 2913.
[7] Chauhan D P, Lapucci C, Geol A et al. Gastroenterology, 1999, 116 (4): 1697.
[8] Abbanat D, Leighton M, Maiese W et al. J Antibiotics, 1998, 51: 296.
[9] Jiang G, Lin Y, Zhou S et al. Acta Scientiarum Naturalium Universitatis Sunyatseni,
2000, 39 (6): 68.
[10] Clerc J T. Structural Analysis of Organic Compounds, Budapost Akademiai Kiado, 1981.
[11] Eaton M A, Hutchinson W D. Tetrahedron Letters, 1971, 18: 1337.
[12] Anthony G, Avent J, Hanson R et al. Phytochem., 1992, 31 (3): 1065.
[13] Anthony G, Avent J, Hanson R. Phytochem., 1992, 31 (3): 3447.