http://www.chemistrymag.org/cji/2001/038037pe.htm |
Aug. 1,
2001 Vol.3 No.8 P.37 Copyright |
Separation and confirmation of matrix materials in logic gate circuit
Li Xiangqing, Wang Xingqiao, Shi Yingyan, Guo Jingfu, Dong Jiangwen,
Liu Guofa
(Department of Chemistry, Jilin University, 119 Jie Fang Road, Changchun 130023,
China)
Received Apr. 18, 2001; Supported by the National Natural Science Foundation of China (Grant No. 20071014)
Abstract Five kinds of
meso-p-hydroxyphenylporphyrins with similar composition and different configuration were
synthesized in one-step. The classical method was utilized to separate the mixture and
obtained six kinds of products. Bands of II-VI were characterized by thin layer
chromatography (TLC), elemental analysis, MS, 1H NMR, UV-vis, IR and XRD,
respectively. The results supported and confirmed the attribution of five bands, band II:
MHTPP, band III: trans-DHDPP, band IV: cis-DHDPP, band V: THMPP, and band
VI: THPP, which are the target products in this experiment. The
meso-p-hydroxyphenylporphyrin isomers purified will be useful for building up oligomeric
porphyrins, which could be used as models for logic gate studies and provide insight into
higher levels of integration in microelectronics.
Keywords Hydroxyphenylporphyrin, Matrix material, Logic gate
The development of new strategies in the synthesis of oligomeric porphyrins, especially those linked by C-C bonds have been synthesized as materials with nanostructure[1,2] and as the models for photosynthesis , photoelectric conversion, information storage and molecular electronic devices[ 3-7]. It is reported [ 8, 9] that four metal dots connected in a square by some metal oxide could serve as logic gate circuit. It is possible that metals in the square are substituted with porphyrin monomers to serve as the smallest building block in logic gate, because of the extensive p -conjugated structure which makes electron transfer faster and easier of porphyrin molecule. Our final approach is to synthesize the envisioned molecular device by different type and definite ratio of hydroxyphenylporphyrin linking with some bidentate ligands, so hydroxyphenylporphyrin isomers to be synthesized and separated are particularly attractive and useful because they are the key reactants in preparing the target products. Few reports about six kinds of porphyrin isomers were synthesized simultaneously in higher yield, separated and characterized, respectively, while these are the specific cases in this paper. The following porphyrin isomers are the functional molecules we obtained: 5-(p-hydroxy) phenyl-10, 15, 20-triphenylporphyrin (H2MHTPP), 5,15-di(p-hydroxy)phenyl-10,20-diphenylporphyrin(trans- H2DHDPP), 5,10-di(p-hydroxy)phenyl-15,20-diphenylporphyrin(cis-H2DHDPP), 5,10,15-tri(p-
hydroxy)phenyl-20-phenylporphyrin (H2THMPP), and 5,10,15,20-tetra(p-hydroxy)phenylporphyrin (H2THPP). In the paper, we describe these preliminary studies aimed at providing matrix materials for assembling logic gate that could play a role in transferring electron at the molecular level and provide more insight into higher levels of integration in microelectronics.1. EXPERIMENTAL
1.1 Materials
Pyrrole (Fluka Chemika-Biochemika) was freshly distilled. The other chemicals were reagent
grade unless otherwise specified. All solvents are purified and dried in accordance with
common procedures.
1.2 Synthesis and purification of mixed meso-p-hydroxyphenylporphyrins
The porphyrin monomers were prepared according to standard literature methods with some modification. p-hydroxybenzaldehyde (8.0 g, 65.6 mmol) and benzaldehyde (6.7 mL, 65.6 mmol) dissolved in 250 mL propionic acid were vigorously stirred and heated at reflux. A propionic acid (20 ml) solution of freshly distilled pyrrole (6.8 mL, 131.2 mmol) was added within 25 min. After an additional 2 h, the reaction mixture was cooled and allowed to stand overnight. Diluted with 800 mL distilled water, adjusted the system to pH = 6 ~ 7 with 6 mol/L aqueous sodium hydroxide, filtration and hot water washing for five times to give a mixture as black and purple powder, dried in a vacuum oven at 80°C for 10 h, then extracted with chloroform until the extracts became colorless, after distilling off the solvents in reduced pressure with a rotary evaporator, afforded 6.6 g (28% yield) of a deep purple crystalline product.
The product was analyzed by TLC and found to be a mixture of six possible porphyrin isomers. 5,10,15,20-tetraphenylporphyrin(H2TPP), 5-(p-hydroxy)phenyl-10, 15, 20-triphenylporphyrin (H2MHTPP), 5,15-di(p-hydroxy)phenyl-10,20-diphenylporphyrin(trans- H2DHDPP), 5,10-di-(p- hydroxy)phenyl-15, 20-diphenylporphyrin (cis- H2DHDPP), 5,10,15-tri(p-hydroxy)phenyl-20- phenylporphyrin (H2THMPP), and 5,10,15,20-tetrakis(p-hydroxy)phenylporphyrin (H2THPP). The crude product was loaded on a neutral aluminium oxide column with chloroform as an eluent to give a bright purple product immediately. The residue was washed with chloroform/ethanol solution, concentrated in a rotary evaporator, and separated further by using silica gel column chromatography with chloroform as eluent initially, gradually with chloroform/ethanol solution of different ratio. Each band collected was subjected to column chromatography once more then TLC, respectively, and five bands purified were obtained at the end. The identities of H2MHTPP, trans- H2DHDPP, cis- H2DHDPP, H2THMPP and H2THPP were confirmed by TLC, elemental analysis, MS, 1HNMR, UV-vis, IR and XRD. Additional analytical data are given in Table 1 ~ 2.
1.3 Analysis and measurements
C, H and N elemental analysis were obtained with a Perkin-Elmer 2400 elemental analyzer. Decomposition temperature was measured with Perkin-Elmer 7 thermogravimetric analyzer and 1700 differential thermal analyzer. Electrospray mass spectra were performed on a Finnigan MAT LCQ electrospray mass spectrometer, electrospray ionization (ESI), spray voltage: 4.5 kV, nitrogen as spray gas, and the samples were injected in a flow rate of 3 m L/min. 1H NMR spectra were carried out on Varian Unity-400 NMR (400 MHz) spectrometers using DMSO as solvent. UV-vis spectra were determined in 1.00 cm matched cell in a UV-2400 spectrophotometer (Shimadzu). Infrared spectra were recorded on a Nicolet Impact 410 FTIR spectrometer in the region 400 ~ 4000 cm-1 using KBr pellets. X-ray powder diffraction spectra were performed on Siemens D5005 X-ray powder diffractometer.
2. RESULTS AND DISCUSSION
2.1 Elemental analysis
Satisfactory elemental analysis of C, H, N were obtained for these products within
permissible limits, MS analysis were consistent with the theoretical results.
Microanalysis, MS and some physical constants are given in Table 1.
Table 1. Elemental analysis, MS and some physical constants of the compounds
Compounds |
Elemental analysis (Calcd.%) |
(M+1)+ |
Yield |
Dec. P. / |
|||
C |
H |
N |
MW |
peak |
(%) |
°C | |
H2MHTPP C44H30N4O |
83.53(83.79) |
4.82(4.79) |
8.74(8.88) |
630.7 |
631.5 |
22 |
400 |
trans- H2DHDPP |
81.46(81.71) |
4.80(4.68) |
8.31(8.66) |
646.7 |
647.5 |
16 |
442 |
cis- H2DHDPP |
81.44(81.71) |
4.84(4.68) |
8.28(8.66) |
646.7 |
647.3 |
19 |
400 |
H2THMPP C44H30N4O3 |
79.27(79.74) |
4.78(4.56) |
8.40(8.45) |
662.7 |
663.5 |
20 |
460 |
| H2THPP C44H30N4O4 | 77.38(77.86) |
4.74(4.46) |
7.98(8.25) |
678.7 |
679.6 |
12 |
434 |
2.2 Electrospray mass spectra
The mass spectra of the compounds II, IV, V and VI (III and IV are isomers) provide the
main fragments at m/z: 554.4, 570.4, 570.5 and 586.6, corresponding to the species C38H26N4O+,
C38H26N4O2+, C38H26N4O2+
and C38H25N4O3+. They could be
attributed to the release of the phenyl, phenyl, phenolic and phenolic group from II, IV,
V and VI, respectively. The (M + 1)+ peaks of II, IV, V and VI were shown at
631.5, 647.5, 663.5 and 679.6, confirming the attribution of the products II~VI.
2.3 1H NMR spectra
1H NMR spectra of the compound II ~ VI have been determined in DMSO using TMS as the internal standard. Chemical shifts are shown in Table 2.
Table 2. 1H NMR spectral data of the compounds
| Compd. | Chemical shift (d , ppm) |
II |
-2.79 (1H×2, pyrrole NH), 7.32-7.34 (2H, m-phenolic), 7.91-7.96 (3H ×3, m, p-phenyl), 8.12-8.14 (2H, o-phenolic), 8.32-8.34 (2H×3, o-phenyl), 8.93-9.04 (2H× 4,b -pyrrole), 10.13 (1H, OH) |
| III | -2.80 (2H, pyrrole NH), 7.32-7.34 (2H ×2, m-phenolic), 7.94-7.96 (3H× 2, m, p-phenyl, 8.12-8.14 (2H×2, m-phenolic), 8.33-8.34 (2H×2, o-phenyl), 8.93 (2H× 2, b -pyrrole),9.02 (2H×2, b -pyrrole), 10.12 (1H×2, OH) |
| IV | -2.80 (1H ×2, pyrrole NH), 7.31-7.33 (2H× 2, m-phenolic), 7.95-7.96 (3H× 2, m, p-phenyl), 8.12-8.14 (2H×2, m-phenolic), 8.33-8.35 (2H×2, o-phenyl), 8.93 (2H×2, b -pyrrole), 9.01 (2H×2, b -pyrrole), 10.11 (1H×2, OH) |
| V | -2.79 (1H ×2, pyrrole NH), 7.32-7.35 (2H×3, m-phenolic), 7.93-7.95 (3H, m, p-phenyl), 8.12-8.15 (2H×3, o-phenolic), 8.32-8.34 (2H, o-phenyl), 8.92 (2H, b -pyrrole), 9.01 (2H×3, b -pyrrole ), 10.11-10.12 (1H×3, OH) |
| VI | -2.78 (1H ×2, pyrrole NH), 7.32-7.34 (2H× 4, m-phenolic), 8.11-8.13 (2H× 4, o-phenolic), 8.99 (2H× 4, b -pyrrole), 10.10 (1H× 4, OH) |
The inner proton (N-H) signals of five
porphyrin isomers were located at the highest field region due to extensive ring current
interaction[10], around -2.79 ppm. The other peaks appeared at low field region
of 7.30 to 10.10 ppm. The resonances of hydroxyl hydrogen occurred at about 10.10 ppm, and
those of pyrrole b
-hydrogens appeared at 8.91 to 8.99 ppm. VI leads to a particularly simple NMR spectrum,
attributed to its high symmetry. The b-protons signals of VI exhibited one singlet at around 8.99 ppm,
while those of III and IV were observed as doublets at the fields of 8.92 and 9.02 ppm.
The 1H NMR results of compounds II ~ VI are in excellent agreement with the
suggested structures.
2.3 UV-Visible spectra
The characteristic absorptions of porphyrin free base are displayed in UV spectra of
compounds I-VI, with a typical set of Soret band and the four Q-bands in the visible
region. The band around 420 nm was assigned to the Soret band which arose from transition
of a1m (
2.4 Infrared spectra
The medium intensity absorptions at about 3050, 3025, 2922, 2850 cm-1 in the spectra of II ~VI remain unchanged according to different number of hydroxyl group, and are attributed to C-H stretches. The strong band observed at about 3413 cm-1 is assigned to –OH stretching vibration, while the absorptions at 3317, 969 and 802 cm-1 are attributed to stretching, bending and rocking vibration of N-H, respectively, which are the characteristic absorptions of porphyrin free base[11]. The medium intensity bands at 1600 and 1500 cm-1 are due to stretching vibration of C = C in the conjugated benzene. The band at 1466 cm-1 is sensitive to the number of hydroxyl group: II shows a strong band at 1466 cm-1, which disappears in III, and shifted by 6, 9 and 13 cm-1 to higher wave numbers in IV, V and VI, respectively. This shows that the substituent and molecular configuration affect on -CH2- shearing vibration. The bands at 1658, 1472, 550 and 490 cm-1 in IV(Fig.1b)disappeared in III(Fig. 1a), while the other peaks between them are almost the same, which indicated that III owns the higher symmetry than IV[12]. So III and IV could be further identified by their contrastive IR spectra (shown in Fig. 1). For the same reason, IR spectrum of VI has the least absorptions, so it possessed the highest symmetry among II ~ VI.
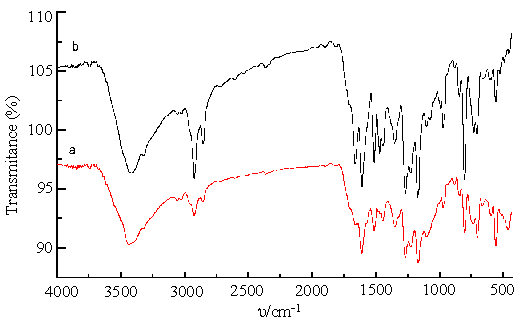
Fig.1 IR spectra of compound III (a) and IV (b)
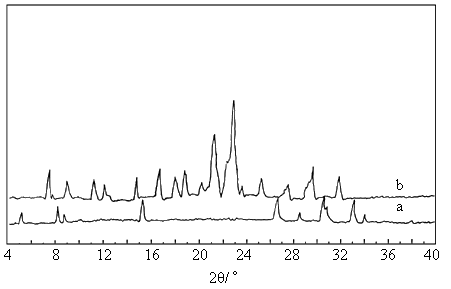
2.5 X-ray powder diffraction spectra
The absorption in III (Fig. 2a) is less than that in IV (Fig. 2b), which could be
attributed to larger inner-extinction in III. In a general way, inner-extinction is
related to symmetry in the molecule, the higher symmetry of the molecule is, the stronger
its extinction is, so the less its absorption is. The result of their XRD spectra is
the same as that of their IR spectra. The XRD spectra of III and IV are shown in
Fig. 2.
3. CONCLUSION
The mixed hydroxyphenylporphyrin isomers were synthesized in one-step and purified by both
column and thin-layer chromatography. The porphyrins purified were identified by TLC,
elemental analyses, MS, 1H NMR, UV-vis spectra, infrared spectra and XRD
spectra. The results confirmed the five bands of II ~ VI, which coincide with theoretical
estimation, II: H2MHTPP, III: trans- H2DHDPP, IV: cis- H2DHDPP,
V: H2THMPP, and VI: H2THPP. On the other hand, the results also
proved that TLC, IR and XRD are good methods for characterization of trans-H2DHDPP
and cis- H2DHDPP by trans-H2DHDPP decrease in polarity and
increase in symmetry relative to cis- H2DHDPP.
The assembly and characterization of the logical gate circuit are in
progress, and are major theme in our laboratory.
REFERENCES
[1] Wang X Q, Gao S, Yu L X et al. Chem. Research in Chinese Universities (Gaodeng Xuexiao
Huaxue Xuebao), 1998, 19 (6): 854.
[2] Segawa H, Wu F P, Nakayama H et al. Synth. Met., 1995, 71: 2151.
[3] Sessler S L, Johnson M R, Lin T Y et al. J. Am. Chem. Soc., 1988, 110 (11): 3659.
[4] Osuka A, Nakajima S, Maruyama K. J. Org. Chem., 1992, 57 (26): 7355.
[5] Kurreck H, Huber M. Angew. Chem., Int. Ed. Engl., 1995, 34 (8): 849.
[6] Kumar R K, Goldberg I. Angew. Chem., Int. Ed. Engl., 1998, 37 (21): 3027.
[7] Nagata T, Osuka A, Maruyama K. J. Am. Chem. Soc., 1990, 112 (8): 3054.
[8] Fulton T A, Dolan G J. Phys. Rev. Lett., 1987, 59 (1): 109.
[9] Orlov A O, Amlani I, Bernstein G H. Science, 1997, 277 (5328): 928.
[10] Collman J P, Elliott C M. Proc. Natl. Acad. Sci., USA, 1977, 74 (1): 18.
[11] Shi T S, Liu B C, Cao X Z. Chem. Research in Chinese Universities (Gaodeng Xuexiao
Huaxue Xuebao), 1991, 12 (8): 995.
[12] Simmons J D, Innes K K. J. Mol. Spectrosc., 1964, 14 (2): 190.