http://www.chemistrymag.org/cji/2001/03c059ne.htm |
Dec. 1,
2001 Vol.3 No.12 P.59 Copyright |
Ma Jinghong, Li Ruifeng, Xie Kechang
(Institute of Special Chemicals, State Key Laboratory of C1 Chemistry and
Technology, Taiyuan University of Technology, Taiyuan 030024, China)
Received Aug. 25, 2001; Supports by the National Natural Science Foundation of China (No. 29973028)
Zeolite NU-87 is a new medium-pore high-silica zeolite[1]. The structure is unique with a two-dimensional channel system, in which the adjacent one-dimensional 10-ring channels are linked by short 12-ring cavities. These bridging sections are accessible only via the 10-ring windows (Scheme 1)[2]. The attractive properties of zeolites are their well organized nanopores and nanochannels which could serve readily as supporting hosts for various metal complexes and metal clusters. A further logical improvement of homogenous catalytic method would consist of the incorporation of the catalyst onto a special inorganic support. The encapsulation of catalytically active metal complexes inside the nanopores of zeolite Y has been believed to be a promising strategies in the development of fine chemical and pharmic catalysts[3]. In the present work, we have successfully accomplished for the first time the preparation of FeSalen complex into the void of new high-silica zeolite NU-87. The resulting complex entrapped within zeolite exhibits good catalytic activity and selectivity for the oxidation of cyclohexene.
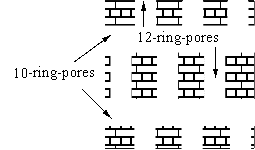 Scheme 1: The channel structure of zeolite NU-87
Scheme 1: The channel structure of zeolite NU-87
The zeolite NU-87 was synthesized according
to the method reported in the literature [4]. The sample was calcined in air at 550°C and
then ion exchanged in 0.1N aqueous solution of NaCl and dried at 120°C to obtain its
sodium cation form. Exchange Na+ for Fe2+ in zeolite NaNU-87 was
carried out in the aqueous solution containing the desired amount of the transition metal
ion (~ 1Fe2+/u.c.). The dried FeNaNU-87 zeolite was mixed with salen ligand
adequately and then sealed in an ampoule. The ampoule was vacuumized and heated at 150°C
until the color of the mixture changed from light yellow into dark yellow when Fe(II)salen
complexes formed. The prepared samples were soxhlet-extracted with dichloromethane and
acetone until no more ligand or metal organic complexes were washed out.
X-ray diffraction analysis showed that the sample containing FeSalen
complexes had the topological structure of NU-87. Uncomplexed salen ligands and FeSalen
complexes have been not observed on the surface of zeolite in the SEM images. The diffuse
reflection UV-vis spectra of the zeolite NU-87 encapsulated FeSalen complexes showed the
geometric distorition of the FeSalen molecule in the peculiar environment(Fig.1), the high
potential complex was intensifying the electron transfer processes and enhancing the
activity of center cation. The special vibrational bands at 200-300nm and ~330nm could be
assigned to the n-p*
transitions and the ligand p->p * transition of phenolic group and azoic group(C=N) respectively.
The bands at 350-450nm was observed arising from metal-to-ligand charge transfer,
originated from dz2->p*, dxz,dyz->p* and dxy->p*. In the IR spectra
of FeSalen complex and NU-87 entrapped FeSalen complex, the qualitative similarities could
be indicated for the characteristic bands at 1600, 1450, 1150 and ~750 cm-1.
Although the zeolitic vibrational absorption bands between 1000-1250cm-1
corresponding to the stretching vibration of SiOSi masked some of the prominent features
of FeSalen molecule, the bands at 1600 cm-1 corresponding to the valence
vibration of the C-C conjugated bonds of the benzene rings and those at 1450 cm-1
corresponding to the C=C stretching vibration of phenyl ring were clearly observed,
respectively. The number of Fe(II)salen complexes in unit cell of zeolite NNU-87 were
determined by the TG-DTA and chemical analyses, to be (Salen)0.5Fe0.6Na1.0
Al3.2 Si64.8 O136.
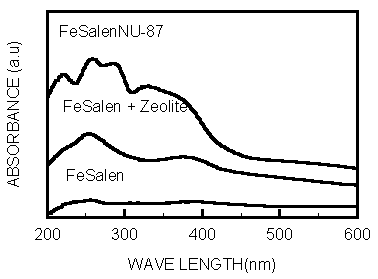
Fig.1 UV-Vis spectra of FeSalen and FeSalenNU-87
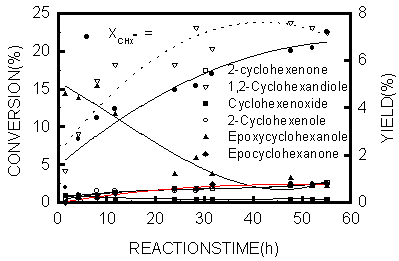
Fig.2 Oxidation of cyclohexene on catalyst
FeSalenNU-87
The activity of the FeSalen complexes entrapped in zeolite NU-87 was tested for the oxidation of cyclohexene in acetone, using H2O2 (30% aqueous solution) as oxidant. The reactions were carried out at 60°C in a glass flask with reflux under stirring. The product distribution was shown in Fig.2. The conversion of cyclohexene on the catalyst of FeSalen complexes encapsulated in zeolite NU-87 reaches to 20% after 50 hours, and the catalyst was of advantage for selective production of cyclohexandiol. The reacted catalyst was washed and dried and characterized, the results revealed that the Fe(II)salen complexes encapsulated in zeolite NU-87 did not decompose and escape during the oxidation reaction.
REFERENCES
[1] Casci J L, Andrews S J. EP APPL. (ICI. Plc), 1990, 377 291.
[2] Shannon M D, Casci J L, Andrews S J. Nature, 1991, 353: 417-420.
[3] Zsigmond Á, Notheisz F, Fráter Z et al. Heterogeneous Catalysis and Fine Chemicals
IV, 453-459.
[4] Glaeser R, Li R, Hunger M et al. Catalysis Letters, 1998, 50: 141-148.