http://www.chemistrymag.org/cji/2003/051004pe.htm |
Jan. 1, 2003 Vol.5 No.1 P.4 Copyright |
Study of protein binding of amlodipine and levamlodipine in human plasma albumin and bovine serum albumin solution by high performance capillary electrophoresis frontal analysis
Zhao Yanyan a,b, Yang Gengliang a,c,
Li Haiying a , Fan Zilin a , Chen Yi c
(aCollege of Chemistry & Environment Science, Hebei University, Baoding
071002; bExperiment Center, Hebei Medical College for Continuing Education,
Baoding 071000; cMolecular Science Center, Institute of Chemistry, Chinese
Academy of Sciences, Beijing 100080, China)
Received Jun. 28, 2002; Supported by the Natural Science Foundation of China (Grant No.20075005) and the Natural Science Foundation of Hebei Province (Grant No. 20077 and No. 202096)
Abstract The protein binding constants,
binding sites and binding rate of Amlodipine and Levamlodipine in Human Plasma Albumin and
Bovine Serum Albumin Solution were studied respectively by High performance capillary
electrophoresis frontal analysis (HPCE/FA). The experiments were carried out by
determining unbound drug concentration in drug-protein equilibrium solution in an uncoated
fused silica capillary (40 cm ¡Á 50 mm i.d.; effective length 32 cm) for capillary electrophoresis under
the following conditions: buffer, sodium phosphate solution (pH 7.4, ionic strength 0.17);
UV detector, wavelength 214 nm; hydrostatic injection mode and the injection time 100 s,
run voltage 10 kV. The unbound drug concentration was calculated from the plateau height
of the profile with good linear relations, r>0.999 for both Amlodipine (N=6) and Levamlodipine(N=6),
respectively. The determination of binding parameters was performed in accordance with
Scatchard and Klotz equation. The reproducibility of the datum determined by HPCE/FA was
confirmed by RSD (RSD < 0.39 %). The capillary electrophoresis frontal analysis
provides an easy, accurate method for pharmacokinetics and pharmacodynamics study of the
drug.
Keywords Amlodipine, Levamlodipine, protein binding parameter, capillary
electrophoresis, frontal analysis .¡¡
1. Introduction
Drug distribution in the body has a direct relation with its selectivity, action duration,
action intensity, effectiveness, toxicity and elimination speed. There are various factors
affecting drug distribution in body and drug plasma protein binding is an important one,
which plays an essential role in pharmacokinetics and pharmacodynamics of the drug.
Protein binding is a rapid and reversible process, where the concentrations of the drug
and the protein easily reach the equilibrium state. Unbound drug in the plasma can
transfer freely to the target organ, producing pharmacological effect, whereas bound drug
hardly passes through the blood capillary walls to reach the action site because of its
bigger molecular weight. In addition, protein binding of a racemic drug is potentially
different from the enantiomers, which may result in the enantioselectivity in disposition
property. The study of enantioselective plasma protein binding is, therefore, essential
for the development of any new racemic drug and for its safety in clinical use.
Amlodipine is a chiral calcium channel antagonist of the
dihydropyridine group and it is currently used in therapeutic and sold on the market. It
is effective for treating hypertension, chronic stable angina, and vasospastic angina. Its
structure is R,
S,2-[(2-aminoethoxy)methyl]-4-(2-chloropheny)-3-ethoxycarbonyl-5-methoxycarbenyl-6-methyl-1,4-dihydropyridine.
Its two enantiomers are S-(-)-Amlodipine and R-(+)-Amlodipine. Levamlodipine is
S-(-)-Amlodipine. Pharmacodynamics and pharmacokinetics study of the two enantiomers of
Amlodipine have been investigated extensively in many reports and it has been found that
Levamlodipine is much more effective than Amlodipine[1] .
Conventional methods used in drug protein binding are the equilibrium
dialysis, ultrafiltration, dynamic dialysis, gel filtration and spectroanalysis [2].
The first three methods involve some problems such as drug absorption onto the
filtra-membrane, leakage of bound drug through the membrane, some constituents in the
membrane dissolved in the solution and Donnan effect (As both the protein and the drug are
charged, the drug concentration on each side of the membrane is not the same.); gel
filtration uses a large amount of protein and spends more time and it is not fit for the
drugs whose bound drug-protein is easy to be cleaved and the spectroanalysis has to remove
protein interference in the determination.
To overcome these difficulties, high-performance frontal analysis
(HPFA) has been developed by using a restricted-access type HPLC column that excludes
large molecule of plasma protein but retains the drug of small molecular size [3].
At present, high performance liquid chromatography frontal analysis (HPLC/FA) has been
widely used in the study of drug protein binding of many drugs [4].
Because of its high resolution power, quickness and small quantity of
sample used, high performance capillary electrophoresis chromatography has become a very
powerful analytical method and has been used in many research fields in recent years. Some
report has also shown its application for the study of protein binding of drug [5].
The purpose of this paper deals with the study of protein binding
constants, binding sites and binding rates of Amlodipine and Levamlodipine respectively in
human plasma albumin (HPA) and bovine serum albumin (BSA) solution by high performance
capillary electrophoresis frontal analysis (HPCE/FA) and analyzed the law of drug-protein
binding.
2.1 Reagents and materials
Amlodipion maleate and Levamlodipion maleate were provided by Shijiazhuang Drug Group(Shijiazhuang, China); HPA was from Shuanglin Drug Co. Ltd., Co.999 group(Zhanjiang, China); BSA (Fraction V) was provided by Baoding HTC Bio-tech Co. Ltd(Baoding, China). The other chemical reagents are all of analytical grade.
Sodium phosphate buffer (pH 7.4, ionic strength 0.17), contains 52.58mmol/L Na2HPO4 and 12.33mmol/L NaH2PO4 prepared with double-distilled water, was used as the background electrolyte solution as well as the solvent of the sample solution and was filtered through 0.45mm filters (Ruili Separation Instrument Factory, Shanghai, China) prior to use.
2.2 Apparatus
The experiment was performed on a Waters Quanta 4000 capillary electrophoresis system (Milford, MA, USA) with a built-in 0 - 30 kV high voltage power supply, a fixed wavelength UV detector near the cathodic end and a forced-air cooling system. Uncoated fused capillary with 50 mm i.d, 40 cm total length and 32 cm effective length was from Yongnian Optical Factory, Hebei Province, China. pH-3C acid meter was from Leici Factory, Shanghai, China. The UV detector wavelength was set at 214 nm and the temperature was set at 30.0 ¡æ. Data processing was carried out with a Waters Millennium 2010 chromatography system. The sensitivity of the detector was set at 0.005AUFS.
2.3 Procedures
A series of Amlodipine-HPA, Amlodipine-BSA, Levamlodipine-HPA and Levamlodipine-BSA solutions of different concentration [(400 - 100£¬350 - 150£¬300 - 200£¬250 - 250£¬200 - 300£¬150 - 350£¬100 - 400) mmol/L ] were made up by using sodium phosphate buffer (pH 7.4, ionic strength 0.17) and the solutions were shaken 100 times/min at 37 ºC for 2h before analysis. The run voltage was 10 kV, and the sample was injected by hydrostatic mode for 100 s. Before each run, the capillary was purged with 0.5mol/L NaOH for 20 min, water for 10min and the buffer solution for 10 min. Between consecutive runs, the capillary was purged with water, 0.5 mol/L NaOH and water for 2 min respectively to obtain good repeatability. The unbound drug concentration (Cf ) was calculated from the plateau height of the profile. The binding parameters were obtained by Scatchard equation and Klotz equation. 3. Results and Discussions
3.1 Effect of voltage
The increase of run voltage can improve the separation efficiency and shorten the separation time. But when the voltage increases, the temperature of the buffer solution in the capillary is increased by Joule heating to result in poor reproducibility. From the experimental results of different run voltage (16 kV, 14 kV, 12 kV, 10 kV, 8 kV, 6 kV, 5 kV, 4 kV), it is found that the best result was given when the voltage is 10 kV.
3.2 Effect of sample injection time (sample volume)
With HPCE/FA, the appearance of a plateau region of drug peak is essential to determine unbound drug concentration, so the sample volume should be large enough to obtain a plateau [6]. When capillary is fixed ( 40cm¡Á50mm I.D.), the peak plateau appears while sampling at about 50s, which is thought as the minimum injection time in HPCE/FA. When the sample injection time is less 50s, a sharp peak appears and when the sample time is prolonged, the peak height increases. When the injection time was above 50s, with the increase of sample volume, the plateau height remains unchanged but its plateau gets wider and wider, even the column gets blocked and difficult in operation When the other conditions are fixed (voltage, temperature and buffer), it is shown that when the injection time was 100s, the reproducibility is the best. Electropherogram in the equilibrium system of Amlodipine and HPA mixed solution was shown in Figure.1.
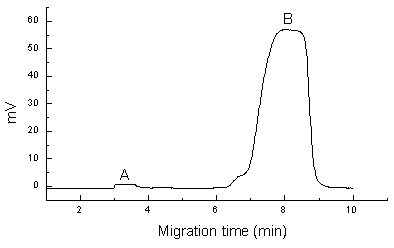
Fig.1 Electropherograms in the equilibrium system of Amlodipine and HPA mixed solution (A: 400mmol/L Amlodipine, B: 100mmol/L HPA)
3.3 Effect of capillary
When the capillary diameter is fixed (50 mm, i.d.), the analysis time is prolonged and its efficiency is
reduced and it is not easy to have plateau zone formed and the reproducibility becomes
poor as the length of the capillary increases. In comparison with the analytical results
of different length of the capillaries (70 cm, 60 cm, 55 cm, 50 cm, 40 cm), it is
concluded that the effect is the best when the capillary length is 40 cm£¨effective length, 32 cm£©and
if it is shorter, it would affect its normal operation. When choosing inner surface
coating capillary, the separating time is much more extended. The good separation effect
and the reproducibility can be obtained when non-coating capillary is used to separate
basic drug from albumin. Because the isoelectric point of albumin is 4.9 and shows
negative charge in phosphate buffer (pH=7.4, I=0.17), basic drug, on the other hand, is
positive, the separation of basic drug and HPA can be carried out by their different
migration direction under the electric field.
3.4 Calibration line
Under the above experimental conditions, the calibration lines was obtained by measuring
the plateau height of a series of Amlodipine and Levamlodipine standard solutions, from 50mmol/L to 500mmol/L. The linear regression equations are :
for Amlodipine, Y = -75.4 + 9.13 X ( r = 0.9994, N = 6)
and for Levamolding, Y = -37.0 + 7.58 X ( r = 0.9995, N = 6 ),
where Y represents the height of the plateau and X the concentration of the sample.
The result shows that there is a good linear relation in the concentration range of 50mmol/L -500mmol/L.
3.5 Determination of the drug and protein binding parameters
According to Scatchard equation [7]
R/Cf=-KR+nK (1)
K and n can be obtained from the slope and intercept of R/Cf vs R curve, where
R is the ratio of the bound drug concentration (Cb) to the unbound protein
concentration (Cp) (R=Cb/Cp), Cf is the
unbound drug concentration, k is the binding constant, and n is the number of the binding
sites on one protein molecule.
Similarly, these parameters can also be obtained by Klotz[8]
equation
1/R=1/n+1/nKCf (2)
When 1/R was plotted against 1/Cf, K and n can be got from the slope and
intercept, where Ct is the total drug concentration.
From the unbound drug concentration of Amlodipine-HPA, Amlodipine-BSA,
Levamlodipine-HPA and Levamlodipine-BSA solution determined by the above method and
Scatchard or Klotz equation, drug-protein binding parameters (K and n) were obtained by
using linear regression and the result were given in Table 1.
Table 1 Drug-protein binding constants and binding sites determined by HPCE/FA ( N = 7)
Association system |
Regression met |
K (M-1) |
n |
r |
Amlodipine-HPA |
Scatchard |
7.30¡Á104 |
2.07 |
0.895 |
Klotz |
7.27¡Á104 |
2.19 |
0.990 |
|
Amlodipine-BSA |
Scatchard |
4.80¡Á104 |
2.02 |
0.904 |
Klotz |
5.18¡Á104 |
2.76 |
0.996 |
|
Levamlodipine-HPA |
Scatchard |
2.16¡Á103 |
1.32 |
0.915 |
Klotz |
2.52¡Á103 |
1.45 |
0.998 |
|
Levamlodipine-BSA |
Scatchard |
1.77¡Á103 |
1.07 |
0.931 |
Klotz |
1.82¡Á103 |
1.29 |
0.999 |
From the results shown
in Table 1, it can be seen that the Amlodipine binding parameters, K and n£¬in HPA solution is extremely close to that in BSA solution and so
is that of Levamlodipine, which shows that the BSA, whose source is more convenient, can
be used as a substitute for HPA in the investigation of drug-protein binding. But there
was great difference between the binding parameters of Amlodipine-protein and
Levamlodipine-protein. In each albumin molecule, there were approximately two binding
sites for Amlodipine (n=2) and one site for Levamlodipine (n=1), and the affinity of two
binding sites for Amlodipine might be different, because the linearity of Scatchard and
Klotz regression equation were not good and their experimental data were inconsistent [8-9].
The binding constant K of Amlodipine-protein is much greater than that of
Levamlodipine-protein, which shows that the affinity of albumin to the racemic drug,
Amlodipine, is stronger than its levo-compound, Levamlodipine. Whether it is one of the
cause of Amlodipine's weaker effect than Levamlodipine's remains to be studied.
Table 2 Drug-protein binding rates of Amlodipine in HPA and BSA solution determined by HPCE/FA
Albumin |
Albumin ( mmol/L) |
Amlodipine£¨ mmol/L£© |
Cf£¨mmol/L£© |
Cb/Ct ¡Á100%(x¡ÀSD, N=4) |
RSD(¡ë) |
(x¡ÀSD) (N=3) |
RSD (¡ë) |
HPA |
3.08¡Á103 |
1.71¡Á103 |
53.7¡À0.47 |
96.9¡À0.03 |
0.57 |
97.1¡À0.22 |
2.27 |
3.08¡Á103 |
1.52¡Á103 |
44.3¡À0.48 |
97.1¡À0.08 |
0.78 |
|||
3.08¡Á103 |
1.24¡Á10 |
33.3¡À1.42 |
97.3¡À0.14 |
1.46 |
|||
BSA |
2.92¡Á103 | 1.71¡Á103 |
44.6¡À1.81 |
97.4¡À0.12 |
1.23 |
97.8¡À0.38 |
3.90 |
| 2.92¡Á103 | 1.53¡Á103 |
28.1¡À1.22 |
98.2¡À0.08 |
0.82 |
|||
| 2.92¡Á103 | 0.95¡Á103 |
21.4¡À1.86 |
97.2¡À0.18 |
1.89 |
Br=Cb/Ct¡Á100% (3)
The binding rates of drug-protein are shown in Table 2 and Table 3. From the results it can be seen that there is no much difference for the binding rates of Amlodipine or Levamlodipine to HPA or BSA.
Table 3 Drug-protein binding rates of Levamlodipine in HPA and BSA solution determined by HPCE/FA
Albumin |
Albumin ( mmol/L) |
Levamlodipine£¨ mmol/L£© |
Cf£¨mmol/L£© |
Cb/Ct¡Á100%(x¡ÀSD, N=4) |
RSD(¡ë) |
(x¡ÀSD) (N=3) |
RSD (¡ë) |
HPA |
3.08¡Á103 |
1.71¡Á103 |
87.8¡À2.92 |
94.7¡À0.47 |
4.92 |
95.0¡À0.28 |
2.99 |
3.08¡Á103 |
1.52¡Á103 |
74.8¡À1.72 |
95.1¡À0.12 |
1.25 |
|||
3.08¡Á103 |
1.24¡Á10 |
64.4¡À2.40 |
95.2¡À0.22 |
2.32 |
|||
BSA |
2.92¡Á103 | 1.71¡Á103 |
36.3¡À0.26 |
97.6¡À0.02 |
0.20 |
97.7¡À0.18 |
1.80 |
| 2.92¡Á103 | 1.53¡Á103 |
34.8¡À0.26 |
97.6¡À0.02 |
0.21 |
|||
| 2.92¡Á103 | 0.95¡Á103 |
22.1¡À0.15 |
97.9¡À0.02 |
0.18 |
This paper presents an effective method to study drug¨Cprotein binding by HPCE /FA. The analysis of drug protein binding law provides a good thinking for the study of drug-protein binding. From the above study it can be seen that HPCE/FA presents an easy, accurate method for pharmacokinetics and pharmacodynamics study of the drug. Acknowledgment This work is supported by funds from the National Natural Science Foundation of China (Grant No. 20075005) and the Natural Science Foundation of Hebei Province, China( Grant No. 200077, 202096). The author Gengliang Yang also wants to gives his thank to the Research Fellowship provided by the Chinese Academy of Sciences.
REFERENCES
[1] Luksa J, Josic D, Kremser M et al. J.Chromatogr. B Biomed. Sci. Appl.
1997, 703: 185.
[2] Zhang Juntian. In Modern Experimental Methods in Pharmacology (Xian Dai Yao Li Shi Yan
Fang Fa Xue). Beijing: Beijing Medical Universary Press, 1998: 1669.
[3] Pinkerton T C. J. Chromatogr. 1991, 544: 13.
[4] Shibukawa A, Kuroda Y, Nakagawa T. J.Pharm. Biomed. Anal. 1999, 18: 1047.
[5] Mohamed N A L, Kuroda Y, Shibukawa A et al. J.Phar.Biomed. Anal., 1999, 21: 1037.
[6] Shibukawa A, Nakagawa T. Anal. Chem., 1996, 68: 447.
[7] Scatchard G, Ann N Y. Acad Sci, 1949, 51: 660.
[8] Klotz I M, Hunston D L. Biochemistry, 1971, 10: 3065.
[9] Feldman H A, Anal.Biochem., 1972, 48:317.