http://www.chemistrymag.org/cji/2003/051003pe.htm |
Jan. 1, 2003 Vol.5 No.1 P.3 Copyright |
(Department of Chemistry and Chemical Engineering, Suzhou University, Suzhou, Jiangsu, China) Received Sep. 3, 2002; Supported by the National Natural Science Foundation of China (No. 20076031) and Jiangsu Science Foundation (BK2002042) Abstract P-p conjugated polyamic acid(PAA) was synthesized by the polycondensation of benzoguanamine (BGA) and pyromellitic dianhydride (PMDA) under microwave irradiation. Then PAA was grafted by lower molecular weight ploy(2-Hydroxyethyl Acrylate)(PHEA) to the main chain. The effect of the amount of catalyst, the molar ratios of PAA and PHEA structural units and the molecular weight of PHEA on the intrinsic viscosity and the graft degree were investigated. Thin films of the graft polymers were obtained by spinning-coat method. The film quality was improved obviously by graft modification. The third-order nonlinear optical (NLO) responses were probed by degenerated four wave mixing(DFWM) technique. The results show that the third-order NLO coefficient of the polymer films is larger than that of the polymer solutions.
Keywords Microwave irradiation, Polyamic acid, Grafting, Third-order nonlinearity
1. INTRODUCTION
The third-order optical materials have attracted considerable interest because of their
potential application in optical switches, modulators, and other all-optical devices. P-
Polyamic acids are a type of excellent third-order NLO materials. However, molecule rigidity and intensive intermolecular force of PAAs make them difficult to be dissolved and melted, which leads to the difficulty of processing. Soft segments grafted on the main chains reduces the intermolecular force, which is an efficient way to improve their solubility and processibility[4].
The study of the use of microwave irradiation in polymerization is a new and interested field[5]. Microwave irradiation has been utilized for the radical polymerization of vinyl monomers and polycondensation of bifunctional monomers[5]. In this paper, benzoguanamine (BGA) and pyromellitic dianhydride (PMDA) were selected as monomers for polycondensation under microwave irradiation. The synthesized PAAs were modified by grafting with low molecular weight ploy(2-Hydroxyethyl Acrylate)(PHEA). Thin films with large third-order NLO response were also obtained. 2. EXPERIMENTAL
2.1 Materials
Benzoguanamine (BGA),98-99%, Shanghai Nanda Chemical Factory; Pyromellitic dianhydride (PMDA) (chemical pure, recrystallized with acetic anhydride, Shanghai Jiaohua Factory) N,N-dimethylformamide (DMF) (analytical pure, refined by reducing pressure distillation after dehydrated with anhydrous Magnesium Sulfate for 24 hours, Shanghai Chemical Reagent Co. Ltd ). 2-Hydroxyethyl Acrylate)(PHEA) chemical pure, refined by reducing pressure distillation. N-methyl-2-pyrrolidone(NMP), chemical pure, refined by reducing pressure distillation. Other reagents and solvents were also obtained commercially.
2.2 Procedure of polymerization
2.2.1 Synthesis of polyamic acid
To a stirred solution of BGA in DMF was delivered nitrogen gas for ten minuters, and then added PMDA in batch. The molar ratio of BGA and PMDA was 1 ̄1. Reaction temperature was controlled at 60ºC by modulating the power of the microwave oven. After irradiation for 2 h, the solution was poured into deionized water, and then the precipitate was separated by filtering, and washed with THF. After filteration, oven drying and grinding, the pale yellow polymer powder was obtained. Fig. 1 is the microwave reaction instrument.
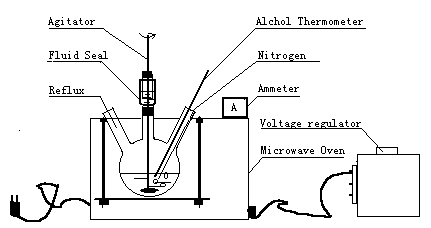 Fig. 1 Microwave
irradiation reaction instrument
2.2.2
Polymerization of HEA
Fig. 1 Microwave
irradiation reaction instrument
2.2.2
Polymerization of HEABenzoyl peroxide(BPO) as initiator was dissolved in isopropanol, then HEA was dripped into it in drops. The reaction temperature was increased to 70ºC, and kept refluxing for 4 hours. The reaction solution was poured to petroleum ether. Then the transparent PHEA separated out. In order to obtain PHEA with different molecular weight, the dosage of BPO were varied (0.4%, 0.6%, 0.8%,1.0% respectively). Based on the characteristic of free radical polymerization, the more the BPO used, the smaller the molecular weight will be.
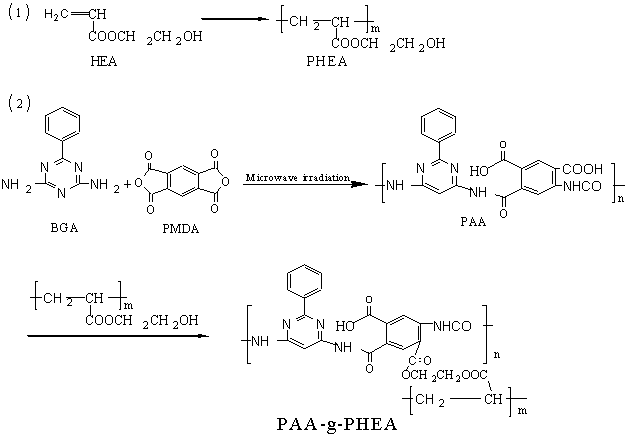
Scheme 1 The route of the reaction
2.2.3 Graft polymerization of PAA and PHEA
PHEA was added to NMP and stirred for 10 hours to dissolve completely. The solution was transferred to three-neck flask. PAA powder was added to the solution. Concentrated sulfuric acid was dripped into the solution at the temperature of 50ºC. Then the oil bath was controlled at 120ºC, and the inner temperature was 115ºC. Water produced in the reaction was separated with oil-water separator. Nitrogen gas was delivered in the whole process. After intensively stirring for 4 hours, the solution was poured into deionized water and stirred to make the remained PHEA to be dissolved. After filtering the polymer was washed with deionized water again and dried at 60ºC to constant weight. The whole procedure is showed in Scheme 1. 3. CHARACTERIZATION AND TESTING
3.1 FTIR spectrum
FTIR spectrum of the polymers was tracked by America Perkin-Elmer 577 infrared spectrometer (KBr pressed disc method). As shown in the Fig. 2, the band at 3442cm-1 may be the vibration absorption of -OH, which is only in the PHEA, the band at 3115cm-1 may be assigned to the stretching vibration absorption of N-H in 每CONH-. The stretching vibration absorption of C=O in 每CONH每 and O每C=O appeares at 1652cm-1, 1610 cm-1. The stretching vibration absorption decrease a little than normal(1750 cm-1) because of the P-p conjugate. 1571.64cm-1 is the stretching vibration of C=C. 1477.67cm-1 is the deformation vibration of the 每CH2每, and its wagging vibration appears at 1268.21cm-1, 1294.41 cm-1. 1168.21 cm-1 is about the stretching vibration absorption of the C每O in the O每C=O. The vibration absorption of C每O in 每CH2OH appears at about 975.10 cm-1. All the above indicate that PHEA has been grafted on PAA.
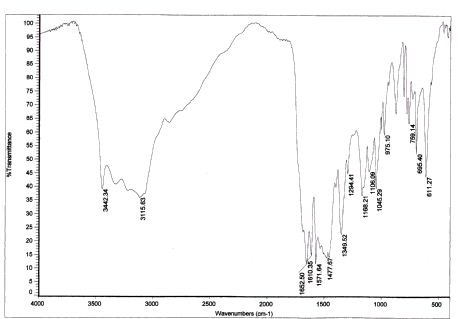
Fig. 2 The FTIR spectrum of the graft polymer
Tab.1 The element analysis results of the polymer
Element |
Percentage in PAA (%) |
Percentage in PHEA-g-PAA (%) |
N |
20.7151 |
17.82851 |
C |
52.10792 |
52.50711 |
H |
4.090433 |
4.490953 |
Elemental analysis of the polymers was carried out with elemental analyzer (Italian 1106-type). The results were listed in Tab. 1. It is observed that the percentage content of nitrogen is reduced from 20.7151% to 17.82851%. The content of carbon and hydrogen decreases in different degree. These changes are related to the change of the polymer structure after grafting.
3.3 The intrinsic viscosity
The intrinsic viscosity measurement of copolycondensate solution was carried out with Ubbelohde viscometer whose inner diameter is 0.7mm. The constant temperature bath was controlled at 30.0㊣0.5ºC, DMF as the solvent.
3.4 Film forming properties
Thin films of PAAs spin-coat on quartz glass substrates and were baked at 60ºC for a quarter. The optimal spinning-coat condition as follows: lower speed stage: 700r/min, 8 seconds; higher speed stage: 2000r/min, 25 seconds. Films quality of polymers synthesized from different monomers molar ratio were compared. It is observed that the films are smooth and even synthesized as the following conditions: molar ratio of PHEA and PAA is 2:1, amount of concentrated sulfuric acid is 0.185g, and the amounts of BPO is 0.4%, 0.6% and 0.8% respectively. That is to say, the molecular weight of PHEA is relatively low.
3.5 Third-order NLO properties
Third-order NLO responses of the polymers were probed by phase conjugated forward three-dimensional DFWM technique. DFWM is a sensitive technique and requires a complicated experimental apparatus as shown in Fig. 3:
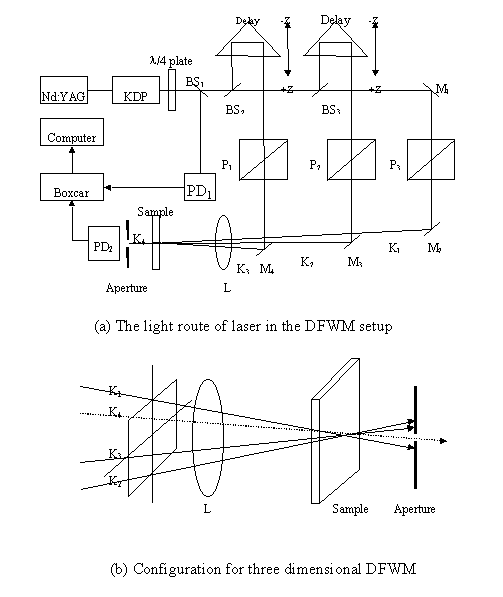
Fig. 3 The scheme of the light route of the DFWM system After passing through a quarter wavelength plate, the pulse laser beam (wavelength: 532nm, pulse width: 35ps, repetition rate: 10Hz and pulse energy about 2.5 mJ) from a frequency-doubled picosecond pulse mode-locked Nd:YAG laser is split into three beams with the same energy by use of reflecting beam splitters. Then temporally and spatially overlapped in the sample with a 205mm focus-length lens (L). The angles between the three beams are about 1o. In the experiment, the intensity of I4 was probed by a PIN photodiode (PD2). The third-order NLO coefficients c(3) can be obtained by comparing the measured signals of the samples with that of carbon disulfide under the same experimental conditions according to the following formula[6]:
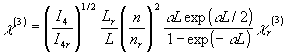 (1)
(1)where n is the refractive index, L is the path length in medium, which is equal to the thickness of the sample cell approximately for liquid sample. Subscript r denotes the reference carbon disulfide and a is absorptivity. cr(3) is 6.8 ℅10-13esu[7]. The response time of samples was obtained by using the formula (2)[8].
Y1/2=2.354s(2)
Where s is standard deviation, which is obtained by Gaussian fitting of DFWM signal curve. Note: The third-order NLO responses of polymers were measured in dilute solution of DMF (0.4g/L). 4. RESULTS AND DISCUSSION
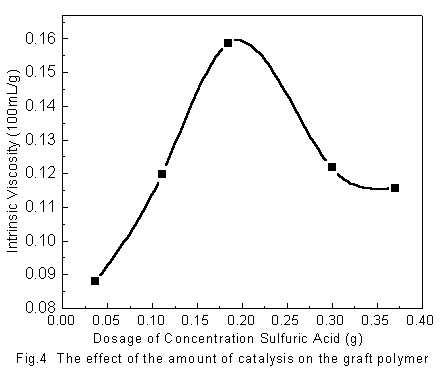
4.1 The effect of the amount of catalyst
Fig. 4 shows the effect of the amount of the concentrated sulfuric acid on the intrinsic viscosity of the graft polymers. It can be observed from the curve that the intrinsic viscosity increased with the increasing of the amount of concentrated sulfuric acid when its amount is relatively small, which shows that increasing of the amount of catalyst can improved the esterification degree. However, when the amount increases to a certain degree, the excess concentrated sulfuric acid can lead to the degradation of the polymers. Therefore, the intrinsic viscosity of polymers is decreasing when the amount of the catalyst increases to a certain degree.
4.2 The effect of the molar ratio of PHEA and
PAA
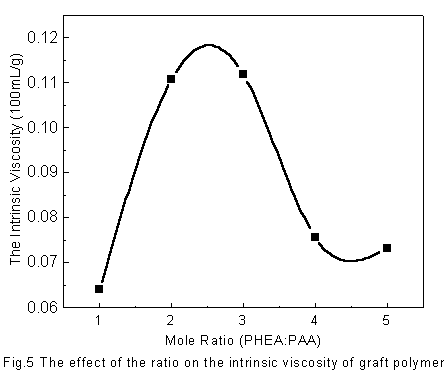
Fig. 5 shows the effect of the molar ratio of PHEA and PAA on the intrinsic viscosity of
graft polymer. It is noted that the intrinsic viscosity of the graft polymers increases
with the increasing of the ratio of PHEA when its proportion is relatively small because
of the increasing of the collision probability of -OH and -COOH. However, when the proportion of PHEA is too large, the
viscosity of the reaction system increases so that the diffusion rate is slow. Thus, the
collision probability is lessening, which leads to the decreasing of the intrinsic
viscosity of the graft polymers. The intrinsic viscosity of the polymers is related to the
graft degree.
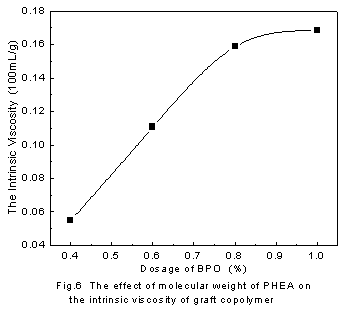
4.3 The effect of the molecular weight of PHEA on the graft polymer
Fig. 6 described the molecular weight of PHEA on the intrinsic viscosity. The curve shows that the intrinsic viscosity of the graft polymers increased with the increasing of the dosage of the BPO, that is, the decreasing of the molecular weight of PHEA. As is known that the shorter chain diffuse readily, therefore, the collision probability of PHEA with PAA molecule is high. In this condition, the graft degree is relatively high.
4.4 Third-order NLO responses of the polymer
films
Third-order NLO responses of polymer films were detected by DFWM technique. The
corresponding solutions were also detected as comparison. The results were listed in Tab.
2, which indicate that the third-order NLO coefficients of polymer solution decrease after
being grafted. Grafting leads to the distortion of the molecular chain, which can decrease
the conjugation degree. So the third-order NLO coefficients reduced. However, the
third-order NLO coefficient of the film sample increases obviously. This phenomenon can be
interpreted that the orientation degree of the films is increased, the molecular
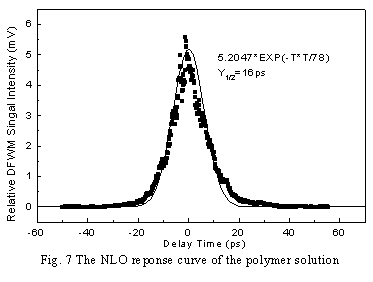
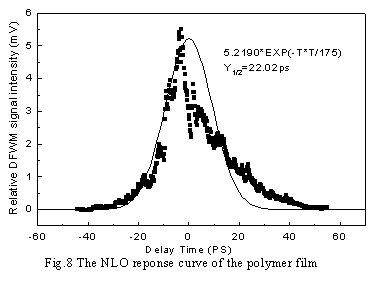
orientation contributes greatly to the third-order NLO response [9]. Fig. 7 and
Fig. 8 are the response curves of polymer solution and film respectively. It is
interesting to note that the NLO curve of polymer solution can be well fitted by a
Gaussian function(solid curve) while that of films cannot be fitted well. This is related
to the light energy loss resulted from the light scattering of the film samples.
Tab.2 c(3) of the polymers in solvent and that of the films (10-13esu)
Sample number |
c(3) of the films |
c(3) in solution |
PAA |
---- |
4.486 |
1 |
5.43 |
4.122 |
2 |
5.79 |
4.312 |
3 |
5.57 |
4.0596 |
The synthesized PAAs have excellent third-order NLO properties, whose maximum third-order NLO coefficient is 4.486℅10-13esu.
Grafting the soft segments to the PAA chains can reduce the intermolecular force, but the solubility and processibility were improved by grafting modification. The third-order NLO coefficient of the polymer increased to 5.79486℅10-13esu after being film formed.
REFERENCES
[1] Sun Z R., Yang X H., Huang Y P., et al. Optics Communications., 1999, 160: 289-291.
[2] Y. Sakai, M. Ueda, A. Yahagi, N. Tanno. Polymer., 2002, 43 (12): 3497-3503.
[3] Wang D., Sui Y., Yin J., et al. J. Funct. Polym., 2000, 1: 35-38.
[4] Lu Q H., Li M., Tao P., et al. J. of ShanghaiJjiaotong Univ., 2001, 35 ( 4): 605-609.
[5] Lu J M., Ji S J., Wu J F., et al. Chemical Journal on Internet., 2001, 3 (8): 41-50.
[6] R. W. Boyd, Nonlinear Optics, San Diego: Academic Press, INC, 1992;
[7] D. C. Donal, Bradley, Y. Mori. Japan. J. Appl. Phys., 1989, 28 (2): 174.
[8] Liu S H., He G S. Intensive Optics and Application (Qiang Guang Guang Xue Ji Ying Yong) Guangdong Science and Technology Press. China.1995.
[9] J. L. Brédas, C. Adant, P. Tackx, A. Persoons. Chem Rev 1994, 94 (1): 243-278.
﹛