http://www.chemistrymag.org/cji/2003/051006ne.htm |
Jan. 1, 2003 Vol.5 No.1 P.6 Copyright |
(a Department of Chemistry, Wuhan University, Wuhan 430072, China; b School of Life Sciences, Wuhan University, Wuhan 430072, China)
Received Oct.1, 2002;
Supported by the National Science Foundation of China. (No. 29972034).Abstract Hydroxyl-Substituted Macrocyclic
Polyamines were synthesized, and two compounds and their metal complexes were found to be
potential antitumor agents in antitumor activity assays against the BEL-7402 (human
hepatocellular carcinoma ) cell line in vitro.
Keywords hydroxyl polyamines complexes antitumor activities
The effective anti-solid tumor drug cis-platinum has been found to form a covalent 1,2-intrastrand adduct at N7 of the guanine base of DNA.[1,2] The design and synthesis of macrocyclic polyamines with catalytic activity and substrate specificity, mimicking natural enzymes, have been found important applications in molecular biology,[3,4] and perhaps in the development of new therapeutics.[5,6] Here we would like to describe briefly the preliminary results of the antitumor activity assays of compound 1, compound 2 and their Co(II), Ni(II), Cu(II) complexes against the BEL-7402 (human hepatocellular carcinoma ) cell line in vitro.
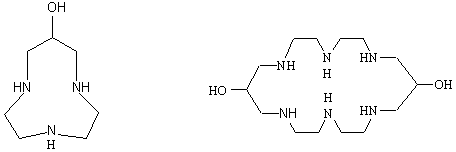
Compound 1
Compound 2
( L 1 )
( L 2)¡¡
In the previous papers
,[7,8] we had reported the synthesis of compound 1 and compound 2. Recently, we got more compound 2 with 4:1 molar ratio for N-tosylated triamines (3) to1,3-dichloro-2-propanol (4). To a stirred solution of compound 3 (22.60g, 0.04mol) in absolute EtOH (500mL) under N2, freshly prepared NaOEt (0.48g, 0.021mol of Na in 100mL of EtOH) was added within 10 min. After 2 h under refluxing, a solution of compound 4 (1.42g, 0.011mol) in EtOH (100mL) was added dropwise over 10 h. The mixture was kept refluxing for 20h. The reaction mixture was cooled, filtered. The washed filtrate was the crude product . The filtrate of EtOH solution was evaporated under vacuum, then CH2Cl2 (25ml) was added, stirred for 2h, filtered. The filtrate was the unreacted 3, it was recovered. The filtered CH2Cl2 solution was evaporated under vacuum and the residue was crystallized from EtOH giving N-tosylated compound 1 (2.15g, 34.6% yield), mp 210-212ºC. [nmax (KBr): 3505, 1334, 1161, 1098 cm-1. dH (CDCl3):2.42(s, 9H), 3.12-3.20(m, 12H), 3.64(s, 1H), 4.45-4.47(q, 1H), 7.23-7.83(m, 12H) ppm. m/z: 623(M++1). Anal. C28H35N3O7S3 Calcd:C, 54.08; H, 5.68; N, 6.76. Found: C, 54.41; H, 5.34; N, 6.45.] The crude product was refluxed in absolute EtOH (500mL) under N2, and the addition of NaOEt (0.01g, 0.004mol of Na in 10mL of EtOH) and a solution of compound 4 (0.26g, 0.002mol) in EtOH (20mL) was repeated, and the reaction was continued for 20 h. The reaction mass was cooled, filtered. The solid was washed with water, EtOH and Et2O to afford white product N-tosylated compound 2 (1.12g, 14.4%yield), mp 280-282ºC. [nmax(KBr): 3522, 1331, 1160, 1091 cm-1. dH (DMSO): 2.42(s, 18H), 3.09-3.26(m, 24H), 3.80(s, 2H), 5.03(q, 2H), 7.42-7.64(m, 24H) ppm. m/z: 1245(M++1). Anal. C56H70N6O14S6. Calcd:C, 54.08; H, 5.68; N, 6.76. Found: C, 53.80; H, 5.49; N, 6.54.]The N-tosyl macrocycle (2 mmol), 33%HBr in glacial AcOH (30 mL) and phenol (2g) were combined and stirred at 80-90ºC for 24 h. After being cooled to rt, the solution was poured into dry Et2O(200 mL) and the red precipitate was recrystallized from EtOH to give the macrocycles 1·3HBr (Yield 85%).[ nmax(KBr): 3340, 3044, 1443, 1101, 1020 cm£1. dH (D2O): 3.18-3.54(m, 12H), 4.54(q, 1H) ppm. Anal. C7H17N3O·3HBr. Calcd:C, 20.02; H, 5.29; N, 10.01. Found: C, 20.27; H, 5.61; N, 9.84] and 2·6HBr (Yield 90%).[ nmax(KBr): 3350, 3050, 1444, 1127, 1023 cm£1. dH (D2O): 3.19-3.54(m, 24H), 4.56(q, 2H) ppm. Anal. C14H34N6O2·6HBr·H2O. Calcd:C, 20.45; H, 5.16; N, 10.23. Found: C, 20.03; H, 5.47; N, 9.89.]
[1+2] condensation product seemed to be found in the careful MS analysis. [crudes (m/z(%):1245(5), 1188(12), 1090(6)); [2+2] product (m/z(%): 1245(M++1)(19), 1090(M++1£Ts)(12))]. It seemed that the preparation of [2+2] product could be considered as a two-step method. Firstly, compound 4 reacted with two molecules compound 3 to give the [1+2] product. Secondly, condensation of [1+2] product with compound 4 yielded the [2+2] product.
These hydroxyl macrocyclic polyamines were versatile chelating agents. They formed stable complexes with transition metal ions.[9,10] The metal complexes for the study were obtained by adding an appropriate amount of a standardised solution of metal ions, to a solution of ligand and buffer.[4]
Two compounds and their metal complexes exhibited promising antitumor activity in vitro, especially against the BEL-7402 cell line. The M:L1 molar ratio was 1:1 for the system M-L1; the M:L2 molar ratio was 2:1 for the system M-L2. After the systems were added with metal ions, the antitumor activity was observably increased. And even when the concentration of systems was diluted to 10-5 mol l-1,the antitumor activity could still be observed. The Cu-L1 system was the most effective for the system M-L1 against BEL-7402 cell line; it was noted that the Ni-L2 system was the most effective for the system M-L2 against BEL-7402 cell line at a concentration of 10-4 mol l-1 and the Cu-L2 system still was the most effective for the system M-L2 against BEL-7402 cell line at lower concentration.
Adherent tumor cells (BEL- 7402) be allowed to adhere for 24 h before the addition of compounds and complexes and were measured by MTT assay.[11,12] The duration of exposure of all tumor cells to compounds and complexes was 48 h. Cell inhibition was expressed in terms of the percentage of control absorbance (¡À1% SD%) following substration of mean blank absorbance in the concentration range 10-3-10-5 mol l-1. The percentages of growth inhibition data of compounds and complexes are shown in Table 1.
Table 1. The percentage of inhibition to BEL-7402 tumor cell lines£¨%£©
Compounds (mol/L) |
10-3 |
10-4 |
10-5 |
L1 |
34 |
16 |
* * |
Co(II) |
79 |
5 |
0 |
Ni(II) |
89 |
3 |
1 |
Cu(II) |
81 |
3 |
2 |
*
It was not measured.In conclusion, two compounds and their metal complexes all exhibited promising antitumor activity in vitro. The percentage of growth inhibition data obtained for the systems M-L1 and M-L2 were similar between them except the Ni-L system.The present results may well be revelent to cleave DNA. Compound 1,2 and their complexes with metal ions and DNA are in progress and detailed results will be reported in due course.
REFERENCES
[1] Takahara P M, Rosenzweig A H, Friederich C A et al. Nature, 1995, 377 (6550): 649.
[2] Sherman S E, Gilbson D, Wang A H J et al. Science, 1985, 230 (4724): 412.
[3] Franklin S J, Current Opinion in Chemical Biology, 2001, 5 (2): 201.
[4] Chand D K, Schneider H J, Aguilar J A et al. Inorg. Chim. Acta, 2001, 316 (1):
71.
[5] Iwata M, Chem. Lett., 1999, 1273.
[6] Iwata M, Bull. Chem. Soc. Jpn., 2000,73 (3): 693.
[7] Wu M H, Cheng G P, He Y B et al. Syn. Commun., 1995, 25 (9): 1427.
[8] Xue G P, Liu Y and Wu C T, Chin. J. Chem., 1998, 16 (6): 536.
[9] Liu S H, Xue G P, Fu E Q et al. Chin. J. Struct. Chem., 1996, 15 (4): 315.
[10] Liu S H, Xue G P, Zhang Z H et al. Chin. J. Struct. Chem., 1997, 16 (5): 355.
[11] Mosmann T, J. Immunol Methods, 1983, 65 (1): 55.
[12] Alley M C, Scudiero D A, Monks A et al. Cancer Res., 1988, 48: 589.
¡¡