http://www.chemistrymag.org/cji/2003/053026pe.htm |
Mar. 1, 2003 Vol.5 No.3 P.26 Copyright |
Application of hydride generation atomic absorption spectrometry with derivative signal processing to direct determination of sub-parts-per billion level of bismuth and tin in water samples
Sun Hanwen, Ha Jing#, Sun Jianmin(Key Laboratory of Analytical Sciences and Technology of Hebei Province, College of Chemistry and Environmental Science, Hebei University, Baoding, 071002; #College of science, Hebei University of Science and Technology, Shijiazhuang 050018, China)
Received Dec.29, 2002.
Abstract A hydride generation atomic absorption spectrometry with derivative signal processing (DHGAAS) had been developed for the direct determination of sub-parts-per-billion level of bismuth and tin in water samples. The signal model and fundamentals of DHGAAS were described. The effects of atomization temperature, carrier flow rate, medium acidity and concentration of KBH4 and KI were investigated and analytical conditions were optimized. The sensitivities for bismuth and tin were 42 and 31 times better than those of conventional hydride generation atomic absorption spectrometry (HGAAS). For a 2 mV min-1 sensitivity range setting, the characteristic concentration was 0.003 mg·L-1 for bismuth and 0.004 mg·L-1 for tin, and the detection limit (3s) was 0.012mg·L-1 for bismuth and 0.010 mg·L-1 for tin, respectively. The proposed method has been applied to the determination of bismuth and tin in real water samples with recovery of 100% and 95.4% for bismuth and tin, respectively.Keywords Derivative, Hydride generation atomic absorption spectrometry, Bismuth, Tin, Water 1 INTRODUCTION
The determination of trace elements has received increasing attention in environmental pollution studies. Particularly, there is an increasing need of a simple, sensitive and accurate method for determining elements at sub-parts per billion (0.xmg·L-1) levels in water. The low level tin of 13.5-32.4mg·L-1 in waste water was determined by graphite furnace atomic absorption spectrometry(GFAAS).[1] Hydride generation has been used for the determination of tin by atomic absorption spectrometry with detection limit of 0.76 mg·L-1[2], but pre-concentration technique is generally required for determination of tin in environmental and biological samples.[3] The concentration of bismuth in leach liquors of ore slages was determined by HG-FAAS. [4] Few paper described for determination of trace bismuth in environmental waters by HGAAS.
Derivative measurement technique based on variation of absorption signal intensity with time had been developed for direct determination of several elements in waters by coupling the laboratory-made derivative measurement system with flame atomic absorption spectrometer.[5] With the use of the derivative technique in FAAS, the sensitivity and detection limit were improved 50-and 10-fold, respectively, compared with those of conventional FAAS. Several methods have been described for the direct determination of Pb and Cd in urine, Cd in waters, vegetables and flours, Pb in water and liqueurs by combining the derivative FAAS with atom-trapping technique.[6-10] The sensitivity was 2-3 orders of magnitude higher than that of conventional FAAS.
A new method was developed for the determination of trace levels of mercury in cosmetic samples, tellurium in urine and selenium in water by hydride generation atomic absorption spectrometry with derivative signal processing. [11-13] The derivative signal model and fundamentals for the determinations of mercury, lead, arsenic and antimony by the derivative hydride generation atomic absorption spectrometry were described.[5, 14,15]
The main purpose of this paper is to establish a derivative signal model and a new method without pre-concentration for the determination of trace and ultra-trace levels of bismuth and tin by hydride generation atomic absorption spectrometry with derivative signal processing. The proposed method has been applied to the determination of bismuth and tin in several water samples. 2 EXPERIMENTAL
2.1 Apparatus
A WFX-YLI atomic absorption spectrometer (Languang Analytical Instrument Factory, Beijing, China) equipped with Bi or Sn hollow cathode lamp (General Research Institute of Non-Ferrous Metals, Beijing, China) was employed for the measurement of the absorbance. A spectral width of 0.2nm was used for the 223.1 or 286.3 nm spectral line. The peristaltic pump and hydride generator were used to introduce the solutions and generate the hydrides, respectively. The generated hydrides were introduced by argon from the generator to a quartz atomizer.
The laboratory-made derivative measurement system consists of two parts, i.e. magnification and differential units. Five sensitivity grades, which correspond to five different magnification levels, expressed as 2, 5, 10, 20 and 50 mV·min-1 , were available in the derivative measurement system. Increase in the derivative signal intensity of the same analyte solution is accompanied by a decrease in the number of the sensitivity grade. The higher sensitivity, i.e. the largest signal intensity, is obtained with the sensitivity grade of the smallest value (2mVmin-1). The derivative measurement system was connected between the spectrometer and a double-pen recorder with which the conventional signals and derivative signals were recorded simultaneously at the 10mV range.
Reagents and chemicals
The bismuth stock solution, 1000mg·L-1, was prepared by dissolving 1.3210 g of bismuth nitrate (Bi(NO3)3·4H2O , analytical reagent grade) in 100ml 15mol·L-1 nitric acid and diluting to1000ml with sub-boiling distilled water. The tin stock solution, 1000mg·L-1, was prepared by dissolving 1.0000 g of metallic tin (high purity grade) in 100 mL of concentrated hydrochloric acid and diluting to 1000mL with sub-boiling distilled water.
Working solutions for determinations of Bi were prepared daily by serial dilution of the stock solution with sub-boiling distilled water. Working solutions for determinations of Sn were prepared daily by serial dilution of tin stock solution with 10% HCl(V/V).
The potassium tetrahydroborate stock solution, 10% (m/V), was prepared by dissolving 10g KBH4 in 100ml 0.2 mol L-1 sodium hydroxide. The solution was kept in a refrigerator and stood for up to four weeks. The working solution of KBH4 was prepared daily by dilution of the stock solution with sub-boiling distilled water.
All the other reagents were of analytical reagent grade. Sub-boiling distilled water was used throughout this work.
Table 1 Recommended experimental conditions
| Parameter | Bi | Sn |
| Wavelength (nm) | 223.1 | 286.3 |
| Lamp current (mA) | 8 | 6 |
| Spectral width (nm) | 0.2 | 0.2 |
| Recorder grade (mV) | 10 | 10 |
| HCl concentration (mol L-1) | 3.0 | 0.1 |
| Carrier flow rate (ml min-1) | 800 | 800 |
| KBH4 solution concentration (%) | 2 | 4 |
The HCl concentration in the sample solutions was adjusted to 3.0 or 0.1 mol·L-1 for determining Bi or Sn. A 5ml of sample solution and 1 mL 10% KI solution were injected to the hydride generator. The carrier flow rate (Ar) was 800ml·min-1, and 0.5ml of KBH4 solution (2% for Bi, 4% for Sn) as reducing agent was pumped into the hydride generator. The generated hydrides were entrained by argon into the heated quartz tube atomizer where the hydrides were atomized immediately. The conventional signal and derivative signal were recorded by the double-pen recorder. The standard calibraton method was used for analysis. The recommended experimental parameters are summarized in Table 1.
3 RESULT AND DISCUSSION
3.1 Mathematical model and characteristic of derivative signal
Derivative hydride generation atomic absorption spectrometric system is shown in Figure 1.
The conventional signals and derivative signals recorded by double-pen recorder are shown
in Figure2.
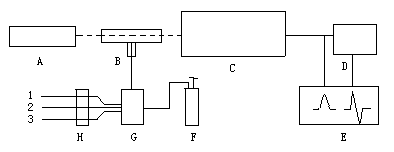
Fig.1 Derivative hydride generation atomic absorption spectrometric system
A: element lamp; B: quartz absorption cell; C: spectrometer; D:derivative measurement system; E: double-pen recorder; F: argon ; G: hydride generator; H: peristaltic pump; 1. KI solution; 2. KBH4 solution; 3. sample solution

Fig.2 The conventional signals and derivative signals The mathematical model for lead signal had been obtained by simulating the signal measured. 12 By using the same method, the mathematical model of the conventional signals for Bi and Sn was obtained as follows:
for the up-side: AH-u =A0 · (1-
for the down-side: AH-d =A0·
Where AH-u and AH-d are the absorbance of the up-side and down-side at different time, A0 is the maximum absorbance. a, b, c and d can be obtained by computer through the investigation of the absorbance values measured for different concentrations(0.04, 0.12, 0.20 and 0.30mg·ml-1 for Bi or Sn) at different times, They are constants within a large range of concentration. For Bi and Sn, a, b, c and d are 1.80, 8.08, 1.01, 26.74 and 1.01, 7.14, 1.58, 24.56, respectively. The results computed on the basis of this model are in good agreement with experimental result.
The derivative signal can be obtained by derivatization of the conventional signal (Eq.1 and Eq.2) with respect to time, it consists of up-peak and down-peak which correspond to the rising edge and falling edge of the conventional signal. The fundamentals of derivative measurement were described in detail in our previous work [14]. 3.2 Linearity of D-HG-AAS
The linearity regression equations and correlation coefficients of DHGAAS at different sensitivity grades for the two elements are listed in Table 2. Table 2 Regression equation and correlation coefficient for DHGAAS
| Element | Derivative grade |
Regression equation |
Correlation coefficient |
| Bi | 20 | A=0.1606 C - 0.0001 | 0.9999 |
| 10 | A=0.3414 C - 0.0004 | 0.9997 | |
| 5 | A=0.7827 C - 0.0001 | 0.9995 | |
| 2 | A=1.818 C + 0.0001 | 0.9998 | |
| Sn | 20 | A=0.0791 C - 0.0002 | 0.9989 |
| 10 | A=0.1441 C - 0.0005 | 0.9992 | |
| 5 | A=0.2663 C - 0.0002 | 0.9998 | |
| 2 | A=0.6692 C+ 0.0002 | 0.9989 |
The output signal of the spectrometer was magnified and then differentiated by the derivative measurement system. Effects of different derivative sensitivity grades on sensitivity, detection limit and precision were studied with bismuth or tin standard solution. The results show that the smaller the number of derivative sensitivity grade (i.e., the higher the amplification), the higher is the derivative absorption signal with the same solution. The derivative sensitivity grade could be selected based on the concentration of the analyte.
Table 3 The sensitivity , detection limit and precision of DHGAAS and HGAAS
Element |
Method |
Derivative range |
Sensitivity |
Detection
limit |
RSD*(%) |
Bi |
HGAAS |
|
0.126 |
0.091 |
2.1a |
|
20 |
0.026 |
0.031
|
2.6c 3.2c 3.1d 4.8f |
|
Sn |
HGAAS |
|
0.125 |
0.098 |
1.9a |
|
20 |
0.025 |
0.034
|
2.2b
|
* based on 7 replicate measurements; The characteristic concentration expressed as sensitivity is defined as the concentration which gives the derivative absorbance of 0.0044. The detection limit is the concentration corresponding to triple the standard deviation of a series of eleven measurements at blank level. The sensitivity, detection limits and precision of DHGAAS and HGAAS are listed in Table 3.
As it is apparent from these data that sensitivities were increased by 42- and 31-fold and detection limits were improved by 10-fold for Bi and Sn, respectively, as compared to conventional HGAAS when using sensitivity range of 2 mV min-1.
3.3.1 Effect of atomization temperature
The temperature of the quartz tube was measured with a thermocouple. High sensitivity and reappearance were attained when the temperature was 850 and 900ºC, respectively, for Bi and Sn atomization.
3.3.2 Effect of acidity condition
The influence of the concentration of hydrochloric acid on derivative absorbance for bismuth and tin was investigated, respectively. The experimental results show that the derivative absorbance for bismuth was higher and constant in the range of 1.5-3mol·L-1 HCl, and for tin increased markedly with increasing acid concentration in the range of 0.02-0.1 mol·L-1 and decreased markedly in the acidity higher than 1.0mol·L-1. A 3.0mol·L-1HCl for Bi and a 0.1mol·L-1 HCl for Sn were selected as optimum acidity conditions in this work.
3.3.3 Effect of KI and KBH4 concentration
As hydride formation is more efficient when elements are present in their lower positive oxidation state, potassium iodide solution was used as pre-reductant for reduction of Bi (IV) in sample solutions to Bi (III). The signals of Bi were doubled by addition of 1ml 10% KI solution. As tin in 0.1 molLl-1 HCl medium was present in Sn (II) state, KI solution would not be used. A 0.5 ml of 2% and a 4% KBH4 solutions were used for generation of bismuth hydride and tin hydride, respectively, with satisfactory results.
3.3.4 Effect of carrier flow rate
The influence of Argon flow rate on the derivative absorbance of bismuth and tin was investigated. The argon flow was used to transfer generated hydride from the hydride generator to the quarts absorption cell. The derivative absorbance was increased with increasing argon flow rate from 200 to 750 ml min-1 because of the introduction of generated hydride from the generator to the absorption cell quickly, and decreased when argon flow rate was higher than 800 ml min-1 because of the dilution atoms in the quarts absorption cell and the reduction residence times of the atoms in absorption cell by the carrier gas. In later experiments, 800 ml min-1carrier gas flow rate was selected.
Sample analysis
Table 4 Determination of bismuth and tin in water samples
|
Bi |
Sn |
|||||||||
Content |
Added |
Found |
Recoverya(%) |
RSDb(%) |
Content |
Added |
Found |
Recovey(%) |
RSDb |
||
Tap water |
0.53 |
0.30 |
0.82 |
98 |
4.2 |
0.45 |
0.30 |
0.73 |
93 |
4.5 |
|
|
|
0.40 |
0.95 |
106 |
|
|
0.40 |
0.83 |
96 |
|
|
Wellwater |
0.37 |
4.8 |
0.25 |
4.9 |
|||||||
| Mineral water | 0.87 |
1.00 |
1.85 |
98 |
3.5 | NDc |
0.08 |
0.078 |
98 |
3.7 |
|
| Mineral water | 0.58 |
4.1 | 0.86 |
3.2 |
|||||||
a: Averages of two replicates
b: 7 replicate measurements for water sample c: Not
detected
There was no interference in the determinations of bismuth and tin with
KI as pre-reductant. The proposed method was applied to the determination of Bi
and Sn in water samples with recoveries of 93-110% and RSDs of 3.0-5.0%. The
results are listed in Table 4.
The derivative hydride generation atomic absorption spectrometry proposed in this paper is a simple, rapid and sensitive method without pre-concentration. The sensitivities for bismuth and tin are increased 42 and 31 times greater than those of conventional hydride generation atomic absorption spectrometry. The present method is suitable for the determinations of trace and ultra-trace level of bismuth, tin and other elements can be formed volatile hydride in environmental and biological samples. Acknowledgements
We express thanks to the Natural Science Foundation of Hebei province, China, for much support to the studied subject. REFERENCES
[1] Wu H, Quan F, Yao M D. Environmental Monitoring, 2001, 13 (3): 383.
[2] Li G F. Environmental Monitoring in China, 1999, 15 (6): 23.
[3] Fang Z. Talanta, 1992, 39 (4): 383.
[4] Liu J, Li G, Qi W Q. China J. Arid Environ. Monitoring, 1995, 9 (3): 132.
[5] Sun H W. Atomic Spectroscopy and Trace Analysis, Hebei University Press, Baoding, China, 2001
[6] Sun H W, Zhang D Q, Yang L L et al. Spectrochim. Acta, Part B, 1997, 52: 727.
[7] Sun H W, Yang L L, Zhang D Q et al. Talanta, 1997, 44: 1979.
[8] Zhang D Q, Li C M, Yang L L et al. J. Anal. At. Spectrom., 1998, 13: 1155.
[9] Zhang D Q, Li C M, Yang L Let al. Anal. Chim. Acta,2000, 405: 185.
[10] Sun H W, Yang L L, Zhang D Q et al. Fresenius. J. Anal. Chem., 1997, 358: 646.
[11] Zhang D Q, Yang L Land Sun H W, Anal. Chim. Acta, 1999, 395: 173.
[12] Ha J, Sun H W,. Sun J M et al. Anal. Chim. Acta, 2001, 448, 145.
[13] Sun H W, Ha J, Sun J M et al. Analytical Sciences, 2002, 18 (5) : 503
[14] Sun H W, Ha J, Sun J M et al Fresenius. J. Anal. Chem., 2001, 371: 1154.
[15] Sun H W, Ha J, Sun J M et al. Anal.Biosnsl.Chem., 2002, 374: 526
กก