http://www.chemistrymag.org/cji/2003/055040ne.htm |
May 1, 2003 Vol.5 No.5 P.40 Copyright |
Li Yiqun
(Department of Chemistry, Jinan University, Guangzhou 510632, China)
The project was supported by the National Natural Science Foundation of China (20272018), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry of China and the Guangdong Natural Science Foundation (974021, 021166)
Abstract A variety of aldehydes can be
converted into 1,1-diacetates efficiently and conveniently in the presence of catalytic
amounts of sulfamic acid at ambient temperature in the room temperature ionic liquid [bmim]PF6
with excellent yields. The ionic liquid is immiscible with water or diethyl ether and can
be reused without noticeable dropdown in activity after separation of the products.
Keywords 1,1-diacetates, catalyze, sulfamic acid, ionic liquid
1. INTRODUCTION
1,1-diacetates are efficient protecting groups for aldehydes as they are stable in neutral
and basic media[1]. Usually, the 1,1-diacetates are prepared from aldehydes and
acetic anhydride in the presence of strong protonic acids such as sulfuric acid[2],
phosphoric acid[2], or methanesulfonic acid[2], and Lewis acids such
as anhydrous zinc chloride[3], phosphorus(III) chloride[4], and
anhydrous ferric(III) chloride[5,6]. Recently the use of montmorillonite clay[7],
TMCS-NaI[8], Sc(OTf)3[9], Cu(OTf)2[10],
PVC-FeCl3[11] SnCl4/SiO2[12], NH2SO3H[13]
etc. as catalysts have also been reported. But these methods have the drawback
as using of organic solvents such as MeCN, MeNO2, Ac2O or
halogenated solvents. It is necessary to develop an alterative solvent for the synthesis
of 1,1-diacetates under mild and environmentally benign conditions.
The use of ionic liquids as a novel class of environmentally friendly
solvents is now of topical interest in the organic synthesis. The ionic liquids have
several unique properties; they can solvate a wide range of organic and inorganic
chemicals, they are highly polar yet non-coordinating and immiscible with a wide range of
organic solvents, and they have a negligible vapor pressure. With the current desire to
avoid the use of volatile organic solvents in organic synthesis, herein, we decided to
investigate the use of potentially cleaner solvents, namely 1-butyl-3-methyl imidazonium
hexafluorophosphate, [bmim]PF6, for the synthesis of 1,1-diacetates promoted by
sulfamic acid. (Scheme 1)
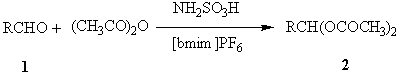
Scheme 1
2. RESULT AND DISCUSSION
The results of the synthesis of 1,1-diacetates in the presence of catalytic amount of
sulfamic acid in ionic liquid [bmim]PF6 are summarized in Table 1. The isolated
yields of the products are excellent in all cases.
Table 1 Conversion of aldehydes to the corresponding 1,1-diacetates catalyzed by NH2SO3H in [bmim]PF6
entry |
Aldehydes (1) | Productsa (2) | Yieldb |
mp ( ºC) |
|
R |
R |
(%) |
Found |
Reported |
|
a |
4-MeO-C6H4 |
4-MeO-C6H4 |
86 |
64-66 |
64-652 |
b |
4-HO-C6H4 |
4-AcO-C6H4 |
92 |
91-92 |
89-902 |
c |
4-HO-3-MeO-C6H3 |
4-AcO-3-MeO-C6H3 |
92 |
75-76 |
79-802 |
d |
(E)-PhCH=CH |
(E)-PhCH=CH |
85 |
82-83 |
84-8512 |
e |
C6H5 |
C6H5 |
91 |
43-44 |
44-4612 |
f |
4-Cl-C6H4 |
4-Cl-C6H4 |
90 |
80-82 |
79-802 |
g |
2-NO2-C6H4 |
2-NO2-C6H4 |
88 |
82-84 |
85.5-86.52 |
h |
3-NO2-C6H4 |
3-NO2-C6H4 |
95(93)c |
64-65 |
63-642 |
i |
4-NO2-C6H4 |
4-NO2-C6H4 |
93 |
125-126 |
124-1252 |
j |
4-Me2N-C6H4 |
4-Me2N-C6H4 |
No reaction |
--- |
--- |
a All the products gave satisfactory spectral analysis for 1H NMR and compared with authentic samples.bIsolated yield. cYields in the parenthesis was repeated in the same batch of the ionic liquids over five runs. |
|||||
As shown the Table 1, the
aldehydes containing the electron withdrawing group have greater activiy than that with
electron donor group and esterification of phenol group was found to produce the
corresponding triacetates under these conditions (entry b and c).
Additionally, it is worth noting that 4-N, N-dimethyl- aminobenzaldehyde failed to produce
the corresponding 1,1-diacetate and the starting material recovered quantitatively after
48h under the same conditions (entry j). This result is the same with
that reported by T. S. Li etal. in the literature[13]. The reason may be
due to that the N, N-dimethylamino group is the strong electron donor group in the compund
1j and hence reduce the activity. The equilibrium between quininoid structure with
aldehyde structure may exist and decreases the activity of the aldehyde group. (Scheme 2)
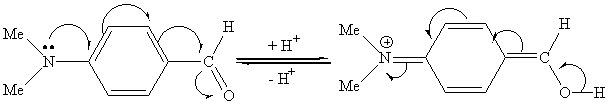
Scheme 2
3. CONCLUSION
The ionic liquid [bmim]PF6 acts as an excellent alternative solvent for the
synthesis of 1,1-diacetates. This is the first report of using sulfamic acid as catalyst
for synthetic chemistry in ionic liquids. The results showed that relatively high activity
of this system reported here.
In conclusion, the present protocol in ionic liquid for the formation
of 1,1-diacetates offers significant improvements over the existing procedures and is an
attractive method of synthesis since the reaction is rapid, the yields are excellent and
the procedure is simple and benign to the environment.
4. EXPERIMENTAL
Melting points were uncorrected. 1H NMR spectra were recorded on a Bruker DRX
400 (400MHz) instrument in CDCl3 with residue CHCl3 at d 7.26 ppm as an internal standard.
Benzaldehyde was purified by distillation. All other chemicals used were of commercial
grade without further purification. [bmim]PF6 was prepared according to the
literature[14].
4.1 General procedure for the preparation of 1,1-diacetates
A mixture of the aldehyde (5.0 mmol), acetic anhydride (15.0 mmol) and sulfamic acid (0.5
mmol) in ionic liquid [bmim]PF6 (2 ml) was stirred at room temperature. The
progress of the reaction was monitored by TLC. After the completion of the reaction, the
saturated sodium hydrogen carbonate solution (10ml) was added. After 10 min the lower
layer [BMIM]PF6 was separated from the aqueous layer which was extracted with
diethyl ether (10 ml¡Á3). The ionic liquid can be reused for the next run by previously
drying for 1h under vacuum at 80ºC. The combined organic layer was washed with brine and then dried
over anhydrous sodium sulfate. Evaporation of the solvent under reduced pressure afforded
the corresponding product that was purified by crystallization from cyclohexane to give
pure 1,1-diacetate.
2a: 1H NMR(CDCl3) dH: 2.11 (s, 6H, 2CH3CO), 3.81 (s, 3H, CH3O),
6.96 (d, 2H, J=6.4Hz, Ar-H), 7.45 (d, 2H, J=6.4Hz, Ar-H), 7.62 (s, 1H, CH).
2b: 1H NMR(CDCl3) dH: 2.13 (s, 6H, 2CH3CO), 2.31 (s, 3H, CH3CO),
7.14 (d, 2H, J=6.4Hz, Ar-H), 7.45 (d, 2H, J=6.4Hz, Ar-H), 7.62 (s, 1H, CH).
2c: 1H NMR(CDCl3) dH: 2.13 (s, 6H, 2CH3CO), 2.32 (s, 3H, CH3CO),
3.81 (s, 3H, CH3O), 7.05 (d, 1H, J=1.6Hz, Ar-H), 7.12 (s, 1H, Ar-H), 7.17 (d,
1H, J=1.6Hz, Ar-H), 7.65 (s, 1H, CH).
2d: 1H NMR(CDCl3) dH: 2.13 (s, 6H, 2CH3CO), 6.20 (dd, 1H, J=16.0
Hz, J=6.4Hz, CH=), 6.87 (d, 1H, J=16.0Hz, CH=), 7.31-7.43 (m, 5H-Ar + CH).
2e: 1H NMR(CDCl3) dH: 2.14 (s, 6H, 2CH3CO), 7.41-7.42 (m, 3H,
Ar-H), 7.51-7.54 (m, 2H, Ar-H), 7.69 (s, 1H, CH).
2f: 1H NMR(CDCl3) dH: 2.18 (s, 6H, 2CH3CO), 7.38 (d, 2H,
J=6.4Hz, Ar-H), 7.46 (d, 2H, J=6.4Hz, Ar-H), 7.63(s, 1H, CH).
2g: 1H NMR(CDCl3) dH: 2.15 (s, 6H, 2CH3CO), 7.59-7.73 (m, 1H,
Ar-H), 7.70-7.73 (m, 2H, Ar-H), 8.05-8.07 (m, 1H, Ar-H), 8.21 (s, 1H, CH).
2h: 1H NMR(CDCl3) dH: 2.17 (s, 6H, 2CH3CO), 7.63 (t, J=8.0Hz,
1H, Ar-H), 7.74 (s, 1H, CH), 7.83 (d, J=8.0Hz, 1H, Ar-H), 8.27 (dd, J=8.4Hz, J=1.2Hz, 1H,
Ar-H), 8.41 (s, 1H, Ar-H)
2i: 1H NMR(CDCl3) dH: 2.17 (s, 6H, 2CH3CO), 7.71 (d, 2H,
J=8.73Hz, Ar-H), 7.74 (s, 1H, CH), 8.28 (d, 2H, J=8.76Hz, Ar-H).
REFERENCES
[1] Green T W, Wuts P G M. Protective Groups in Organic Synthesis, 2nd Ed, John Wiley, New
York, 1991, p178 and 184.
[2] Freeman F, Karchefski E M, J. Chem. Eng. Data., 1977, 22: 355.
[3] Scriabine, I. Bull. Soc. Chim. Fr., 1961, 1194.
[4] Michie J K, Miller J A. Synthesis, 1981, 824.
[5] Kochhar K S, Bal B S, Deshpande R P, Rajadhyakisa S N, Pinnick H W. J. Org. Chem.,
1983, 48: 1765.
[6] Jin T S, Du G Y, Zhang Z H, Li T S. Synth. Commun., 1997, 27: 2261.
[7] Zhang Z H, Li T S, Fu C G. J. Chem. Res. (S), 1997, 174.
[8] Deka N, Borah R, Kalita D J, Sarma J. J. Chem. Res. (S), 1998, 94.
[9] Aggarwal V, Fonquerna S, Vennal G P. Synlett., 1998, 894.
[10] Chandra L, Saravanan P, Singh V K. Synlett., 2000, 359.
[11] Li Y Q, Synth. Commun., 2000, 30: 3913.
[12] Li Y Q, Cheng L H. Chin. Chem. Lett., 2001, 12: 565.
[13] Jin T S, Sun G, Li Y W, Li T S. Green Chem., 2002, 4: 256.
[14] (a) Bonhôte P; Dias A P, Papageorgiou N, Kalyanasundaram K, Grätzel M.
Inorg. Chem. 1996, 35: 1168.
(b) Suarez P A Z, Dulius J E L, Einloft S, de Souza R F, Dupont J. Polyhedron 1996, 15:
1217