http://www.chemistrymag.org/cji/2003/057056pe.htm |
Jul. 1, 2003 Vol.5 No.7 P.56 Copyright |
(School of Science, Tianjin University, Tianjin, 300072, China)
Received Mar. 4, 2003; Supported by the National Natural Science Foundation of China (No.59772019)
Abstract Heterogenous photocatalysed
reduction of aqueous Na2CO3 has been carried out by using nano LaCoO3
powders as a semiconductor. Formic acid and formaldehyde were identified as the
photoproducts spectrophotometrically using Nash reagent. The effect of the various
parameters such as Sodium Carbonate concentration, the amount of photocatalyst and
different light sources on the yield of the photoproducts was also investigated. It shows
that nano LaCoO3 has photocatalytic activity. Irradiation leads to the
production of electrons in the conduction band of the LaCoO3 semiconductor. It
is likely that the photoproduced electrons initially reduce CO32- to
HCOO-, and then to HCHO and CH3OH.
Keywords photocatalysed reduction, sodium carbonate, nano particles, LaCoO3
1 INTRODUCTION
The increase in carbon dioxide has resulted in some serious environment hazards which
have already alarm for future generations. Hence, attention has been raised to carry out
an intense search of an alternate environmental friendly technology for energy production.
The reduction of carbon dioxide using semiconducting powders as
photocatalysts has been reported by Aurian-blajeni [1], TiO2 and
SrTiO3 are known for their high stabilities towards photo corrosion and their
favorite band energies[2]. Possible ways to extend the semiconductor photo
response to light absorption involve doping with suitable foreign cations[3]
and sensitizing by coating with photoactive dyes [4].
A detailed survey of literature reveals that little attention has been
paid to the use of nano complex oxides LaCoO3 semiconductor in aqueous media
for the photocatalytic reduction of carbon dioxide. The present work on this topic was
therefore undertaken.
2 EXPERIMENTAL
All the reagents were Analar grade. The LaCoO3 particles were prepared by a
citrate method.
50mL of 0.1mol·L-1 lanthanum nitrate and 50mL of
0.1mol·L-1cobalt nitrate were mixed in 60mL of 0.5mol·L-1
citric acid (mole ratio of La: Co: citric acid =1:1:6)
under magnetic stirrer. The mixture was evaporated under infrared lamp (250W) to become a
viscous liquid. The next drying was carried out at 95
The crystal structure of catalyst was examined on an X-ray diffraction instrument (Rigaku Geigerflex-2038) using monochromatic Cu Ka radiation.
The photoacoustic spectra of LaCoO3 were recorded on an OAS-400 photoacoustimeter with a scanning range of 300nm-3.2m m.
The photocatalysed reduction of aqueous Na2CO3 was carried out in a 250mL tapered bottle containing 100mg of LaCoO3 and 50mL of 0.01 mol·L-1 Na2CO3. The bottle was set on a vibrator operated at 120 times per minute. The whole reaction mixture was exposed to the light of different intensity. For this purpose a 450W fluorescent Hg lamp or sunlight at 9:00-10:00 a.m. in the mid- September of Tianjin were used as the irradiator. The distance between the bottle and lamp was 25cm. The temperature was attained by a bath cooler to prevent the photo reduced products from evaporation.
The amount of formaldehyde and formic acid were determined spectrophotometrically using Nash reagent at lmax 412nm. Nash reagent was prepared by adding 150.0mg of ammonium acetate, 3.0mL of acetic acid and 2.0mL of redistilled acetyl acetone in 1L of water. The reagent was kept in the dark to avoid any photochemical reaction.
For measurement of the concentration of formaldehyde, the test solution (0.5mL) was taken in a test tube and 2.0mL of Nash reagent was added. The mixture was heated for 5 min in a water bath at temperature of 50-60ºC. A bright yellow color was developed in the solution. This solution was then allowed to cool at room temperature and then the optical density was measured at lmax 412nm. The concentration of formaldehyde in the test solution was determined using a calibration curve. The concentration of formic acid was measured by an indirect manner using Nash reagent. First, the formic acid was converted into formaldehyde by treating it with magnesium and hydrochloric acid. Other product was not measured.
3 RESULTS AND DISCUSSION
According to the XRD spectrum of the prepared LaCoO3 (Figure 1), the crystal
lattice parameters of LaCoO3 were calculated (Table 1). Compared to the
theoretical values (No: 25-1060), we can find that the experimental value is well
consistent with the theoretical one, which suggests that the prepared LaCoO3 is
a pure phase compound. By the TEM photography (Figure 2), the particle size of LaCoO3
is 40-50nm.
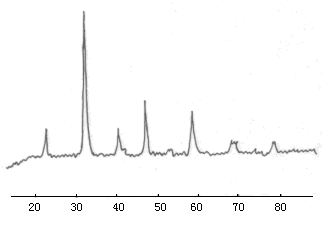
Fig 1
XRD spectrum of LaCoO3 (
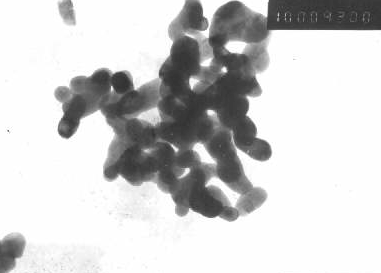
Fig 2 The TEM photograph of catalysts (×105)
Table 1 The theoretical and experimental values of crystal lattice parameters of LaCoO3
Theoretical values |
3.820 |
2.719 |
2.217 |
1.912 |
1.564 |
1.360 |
Experimental values |
3.834 |
2.714 |
2.222 |
1.918 |
1.566 |
1.360 |
3.1 Effect of photocatalyst concentration
The dependence of HCHO and HCOOH yields on the amount of LaCoO3 was
investigated and the results are summarized in Figure 3.
It can be seen that an increase in the amount of photocatalyst results
in the increase of the yields of both HCHO and HCOOH. This increase may be attributed to
the exposed surface area of the reaction vessel, which is completely covered by a specific
number of LaCoO3 particles. However, after a certain limit (100mg) has been
reached, there is no increase in the exposed surface area and hence also in the yield.
There is a saturation point above which any increase in the amount of photocatalyst has
negligible effect on the yields of HCHO and HCOOH.
This may also be explained on the basis of geometry of the reaction
vessel. This was confirmed by taking reaction vessels of different geometry and it was
observed that the saturation point was shifted to a higher value when reaction vessels of
larger capacity were used. On the other hand, the plateau was observed when small reaction
vessels were used.
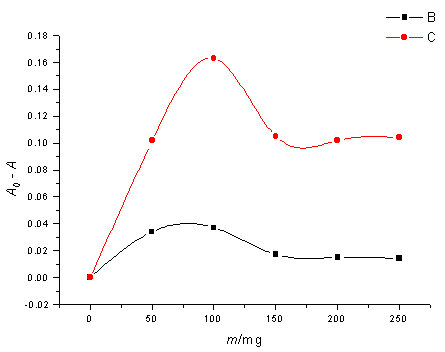
Fig 3 Effect of amount of catalyst on the yield of reduced products(C:HCOOH, B:HCHO)
3.2 Effect of different light sources
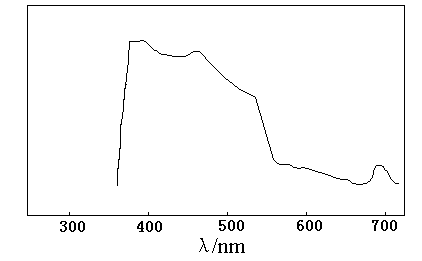
The effect of light sources on the formation of HCHO and HCOOH was also investigated using high pressure mercury lamp and solar irradiation. The results are reported in Table 2. It was also observed that higher yields of HCHO and HCOOH were obtained under solar irradiation than upon the mercury lamp. This may be explained on the basis that the range of wavelength of solar light is larger than mercury lamp (>410nm), while the absorption peaks of LaCoO3 situated in ultraviolet region and visible light region (Figure 4). When the suspensions are irradiated with sunlight, the number of photons per unit area striking the catalyst increases and there is a corresponding increase in the photoreduction of carbonates to formaldehyde and formic acid.
Table 2 Effect of different irradiator on the yield of reduced products formaldehyde and formic acid
Irradiator |
HCHO |
HCOOH |
A0-A content×104/(molL-1) |
A0-A content ×104/(molL-1) |
|
Sunlight |
0.161 3.60 |
0.18 4.07 |
Mercury lamp |
0.038 0.86 |
0.17 3.85 |
REFERENCES
[1] Aurian-blajeni B, Halmann M, and Manassen J. Sol.Energy, 1980, 25:165.
[2] Mills A, Porter G. J.Chem.Soc., 1982, 78:3659.
[3] Malati M A, Wong W K. Surf. Technol., 1984, 22: 305.
[4] Fan F F, Bard A J. J. Am. Chem. Soc., 1979, 101: 6139.
[5] Fu X X, Yang Q H, Wang J Zh et al. Journal of Chinese Rare Earth (Zhongguoxituxuebao),
2002, 20 (6):689.
Na2CO3在纳米LaCoO3体系中的光催化还原
崔晓亮 杨秋华 傅希贤
(天津大学理学院,天津300072)
摘要 以纳米半导体粉末LaCoO3为催化剂进行Na2CO3水溶液的光催化还原实验。采用Nash试剂以分光光度法检测光还原产物甲酸和甲醛。研究Na2CO3浓度、催化剂用量和不同光源对光还原产物产率的影响。结果表明纳米LaCoO3具有光催化还原活性。辐射导致LaCoO3导带中产生光生电子。光生电子首先将CO32-还原为HCOO-,进而还原为HCHO和CH3OH。
关键词 光催化还原;碳酸钠;纳米粒子;LaCoO3