http://www.chemistrymag.org/cji/2003/057055pe.htm |
Jul. 1, 2003 Vol.5 No.7 P.55 Copyright |
Study on binding of
clozapine to serum and plasma proteins by capillary electrophoresis-frontal analysis
Zhou Dawei, Li Famei
(School of Pharmacy, Shenyang Pharmaceutical University, Shenyang 110016, China)
Received Apr.21, 2003; Supported financially by the National Natural Science Foundation
of China (Grant No. 29975018).
Abstract Capillary electrophoresis in the frontal analysis mode
(CE/FA) was developed to determine the unbound clozapine concentration in Human Serum
Albumin ( HSA ) solution, human plasma, rabbit serum and plasma in in vitro experiment.
Samples were injected directly into an uncoated fused-silica capillary (65cm¡Á75mm i.d.; effective length of 35cm ) and
the separation was accomplished within 11 min without extensive sample pretreatment. The
most suitable running buffer to separate unbound clozapine from endogenous substances was
found to be 67mmol L-1 phosphate buffer of pH 7.4 containing 1mmol L-1
EDTA and 0.5mol L-1 glycine. The unbound clozapine concentration was directly
measured from the height of the frontal peak. The methodology was comparable with good
correlation to the conventional ultrafiltration method. It was found that clozapine is
bound in human plasma, rabbit plasma and serum strongly, while weakly bound to human serum
albumin. The present method enables the determination of unbound drug concentration in
multiple equilibria system with sample volume of nl level, and would be useful especially
for the study on protein binding in biological samples available in minute quantity.
Keywords clozapine, HPCE-FA (high
performance capillary electrophoresis-frontal analysis), protein binding, serum and plasma
1. INTRODUCTION
The overall protein binding of a drug involves multiple equilibria, which all together
affect the unbound ( free ) concentration in the blood and thus, the distribution,
metabolism, elimination and pharmacological property. There are numerous methods
applicable to studying on drug binding to plasma proteins [1-5]. Current high
performance capillary electrophoresis (CE) is the most dynamically growing analytical
technique in studying molecular interactions because of its high speed, efficiency and
selectivity. CE offers several modes for the quantitative assessment of protein-drug
interactions depending on the stability of the complex or the on-and-off kinetics of
binding reaction[6]. If the mobility of the protein is equal to that of the
complex, capillary electrophoresis-frontal analysis (CE-FA) is the most favorable method
among them to investigate molecular interaction[7].
The CE-FA method is simple, robust, easy to implement, can deal with
multiple equilibria and requires less material. In addition, the results of the CE-FA
method agree very well with those obtained with conventional methods [7]. The
CE-FA method has been successfully applied to the investigating the interaction of drugs
with human serum albumin (HSA) [8], plasma lipoproteins[9], a1-acid lipoprotein[10],
human plasma[11], etc. The principle and main features of CE-FA method were
described in the literature[7].
Clozapine (Fig. 1), a tricyclic neuroleptic and antidepressant, is
highly protein-bound in serum[12]. It has been proven to be very effective in
the treatment of therapy resistant psychotic patients and patients who are suffering from
the extra pyramidal side-effects ( EPS ) of the classic antipsychotics. It was reported
that the binding affinity of clozapine to a1-acid glycoprotein (AGP) is stronger by two orders of
magnitude than that to HSA [13]; the overall human plasma protein binding
property of clozapine has been investigated by equilibrium dialysis followed by HPLC[12].
The present study investigated the determination of unbound Clozapine
in HSA solution, human plasma, rabbit plasma and serum samples by CE-FA method in in vitro
experiment. The factors affecting the electrophoretic separation and the applied
foreground of the present method were discussed.
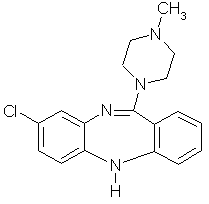
Fig.1 Chemical structure of clozapine.
2. EXPERIMENTAL
2.1 Reagents
Human serum albumin ( HSA, fatty acid free ) was purchased from Sigma ( St. Louis, MO, USA
). Clozapine was supplied by the Institute of Pharmaceutical and Biological Product
Control (Beijing, China ). All other chemicals were of analytical grade and obtained from
Shenyang Chemicals (Shenyang, China). The water was always twice distilled. Phosphate
buffer (67mmol L-1, I=0.17mol L-1) of physiological pH (7.4) was
used as the sample solvent. Uncoated fused silica capillary (75mm i.d.) was purchased from Ruifeng Chromatographic Product Co. (
Yongnian, Hebei, China ).
All CE experiments were performed in HV-301 HVPS Capillary
Electrophoresis System ( Younite Unimicro Technologies, Co. Ltd, Tongliao, Inner Mongolia,
China ) with a VUV-22 UV detector fixed at 214nm. The power supply was operated in
voltage-controlled mode. The operating parameters of CE are given in Table I. The
electrophoretic data were acquired with a Jiangshen workstation ( Dalian, China ).
Table 1 Operating parameters of CE
| Parameter | Setting |
| Fused-silica Capillary | 0.65m(Lc)¡Á75mm i.d; LE=0.35m |
| Sample injection | Hydrostatic injection; Dh=0.11m, 30s |
| Detection | UV at 214nm |
| Voltage applied | -6kV |
| Temperature | room temperature |
| Buffer solution (BGE) | Potassium Phosphate buffer (pH
7.4, 67mmolL-1) containing 0.5molL-1 glycine and 1mmolL-1 EDTA |
2.2 Preparation of serum and plasma
The rabbit blood was drawn into tubes containing potassium oxalate and sodium
fluoride as anticoagulant and immediately centrifuged, and the plasma was separated; Serum
was obtained in tubes with no anticoagulant, the blood was allowed to clot at room
temperature for 20min and then centrifuged. The plasma and serum were divided into
aliquots and stored frozen until used in the binding experiments.
Pooled human plasma from healthy donor was collected on CPD ( citrate-phosphorus-dextrose
) in PVC bags from Medical Macromolecular Manufactory (Tianjin, China ).
2.3 Preparation of samples
The running phosphate buffer was prepared by mixing 10 parts of 67mmol L-1
dipotassium hydrogen phosphate and one part of 67mmol L-1 potassium dihydrogen
phosphate, EDTA and glycine were added to the mixture at a concentration of 1mmol L-1
and 0.5mol L-1, respectively; Isotonic phosphate buffer was prepared by mixing
10 parts of 67mmol L-1 dipotassium hydrogen phosphate and one part of 67mmol L-1
potassium dihydrogen phosphate with the isotonicity of each solution being adjusted with
approximately 5 and 4mg/ml of NaCl, respectively, yielding an ionic strength of 0.17mol L-1
and pH 7.4. The obtained running phosphate buffer and isotonic phosphate buffer were
degassed by sonication in ultrasonic water bath ( DL-180, Shipuhaitian Electronical
Instrument Co., Xiangshan, Zhejiang, China ) for 25min and filtered through a 0.45mm membrane filter before use. HSA
solution of (550mmol L-1)
in 67mmol L-1 isotonic potassium phosphate buffer ( pH7.4; I=0.17mol L-1)
was prepared by adding the appropriate volume of buffer to a weighed amount of HSA.
Clozapine was dissolved in methanol providing as a stock solution. Five 50ml aliquots stock solution were pipetted
into five labelled tubes, evaporated under N2 stream to dryness, and
redissolved in 1ml isotonic phosphate buffer, HSA solution, human plasma, rabbit plasma
and serum, respectively. The tubes were gently shaken at room temperature for 2h to
dissolve clozapine. The equilibrated sample was analyzed for total or unbound clozapine
concentration by direct injection into the CE system. At the same time, half volume of the
sample was used for preparation of ultrafiltrate which was then assayed for unbound
clozapine concentration with CE.
2.4 Determination of unbound clozapine by CE-FA
Uncoated fused silica capillary (65cm¡Á75µm i.d, effective length of 35cm ) was
filled with phosphate buffer (pH 7.4, containing 0.5mol L-1 glycine and 1mmol L-1
EDTA ). The column temperature for separation was maintained at room temperature. The UV
detector was set at 214nm. The samples were introduced into the separation capillary
hydrostatically for 30s with a height of 0.11m between two ends. The injection end of the
capillary was then immersed into the running buffer and a voltage of -6kV was applied at
both ends. The capillary was cleaned between runs by running 30mmol L-1 SDS for
8min and running buffer for another 8min. The unbound concentrations of clozapine were
determined by comparing the plateau height in the electropherograms of the equilibrated
samples with that of neat clozapine solution (standard ).
2.5 Determination of unbound clozapine by ultrafiltration
Ultrafiltration using Microcon Centrifugal Filter Device with a MW cut-off of 10,000 (
Millipore, USA ) was employed to determine the unbound drug concentration. Membranes were
pre-treated by centrifuging 0.2 ml of 0.1mol L-1 sodium hydroxide followed by
rinsing with twice distilled water (0.5ml ), and then the sample solution of 500ml was transferred into the sample
reservoir, equilibrated for 10minutes, and centrifuged at 2000g with a fixed angle rotor
for 8min at room temperature. The ultrafiltrate of about 100nl was injected and analyzed
by CE-FA method described above. Possible absorption of clozapine to the filter was
investigated by measuring filtration of a solution in buffer at 50mmol L-1.
2.6 Data analysis
All statistical analyses were performed using the software package SAS ( Version 8.0, SAS
Institute Inc. Cary, NC, USA.). Comparisons of the means between the two methods were
performed by the paired t test and an associated p<0.05 was considered significant. An
ANOVA test was also used to compare the mean valuesof unbound clozapine concentration in
different samples.
3. RESULTS AND DISCUSSION
3.1 Electropherogram Characteristics
In the frontal analysis the injected sample volume must be large enough in order to form a
plateau peak. The effect of sample injection time on the plateau formation was
investigated in this study. The mixed solution of 200mmol L-1 clozapine and 550mmol L-1 HSA was injected for
different injection time (5, 7, 10, 15, 20, 25 and 30s ). The unbound clozapine showed an
ordinary electrophoresis peak when the injection time was shorter than 10s. A plateau peak
formed with an injection time of 10s. While the peak width became broader with further
increasing injection time, the plateau height was almost unchanged regardless of the
injection time. The injection time was set at 30s in this assay.
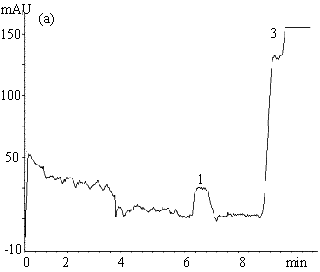
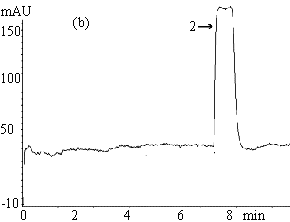
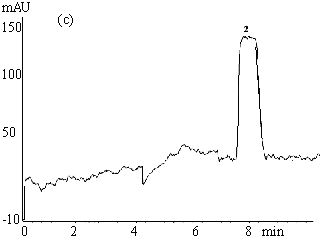
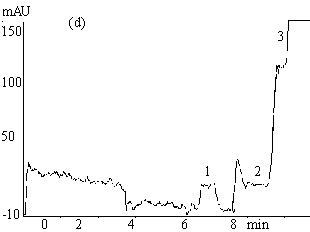
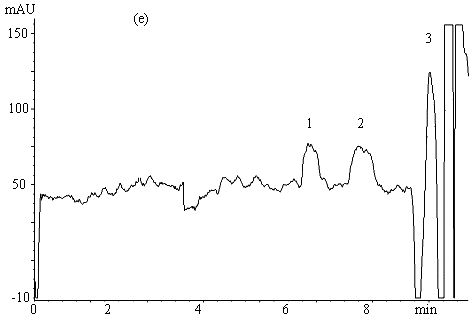
Fig.2 Electropherograms of blank rabbit plasma (a) ; 200mmolL-1
clozapine (b) ; 200mmolL-1 clozapine in 550mmol L-1 HSA
solution (c) ; 200mmol L-1 clozapine in rabbit plasma (d) ; ultrafiltrate
of 200mmol
L-1 clozapine in rabbit plasma (e) .
Electrophoresis conditions: 67mmol L-1 phosphate buffer ( pH 7.4 containing
1mmol L-1 EDTA and 0.5mol L-1 glycine ) , Capillary length 0.65m (
0.35m to the detector ) , detection 214nm, -6kV, 30s hydrostatic injection (0.11m high).
1 and 3 endogenous substances, 2 free clozapine.
Typical electropherograms
obtained under the optimum condition for the separation of clozapine from the endogenous
interference in plasma and serum are shown in Figure 2. The migration time for clozapine
was between 7.5-8.5min. The total electrophoretic run time was 11min. Fig. 2( a )-( e )
successively show electropherograms of blank rabbit plasma, 200mmol L-1 clozapine solution
(the plateau height represents the total drug concentration), 200mmol L-1 clozapine in 550mmol L-1 HSA
solution, 200mmol
L-1 clozapine in rabbit plasma sample and its ultrafiltrate. Peak 1 and 3 (Fig.
2a, d, e ) are from the endogenous substances detected in the plasma or serum. The former
might be a compound of small molecule while the latter macromolecules such as proteins.
HSA, however, migrated slower (20-23.5min) and is not shown in Fig. 2c. The plateau peak 2
appeared in Fig. 2b-e is clozapine, the height of which reflects the unbound clozapine
concentration in the samples. It is obvious that unbound clozapine was separated well from
endogenous substances, and gave a clear and wide plateau.
3.2 Effects of separation voltage and composition of running buffer on the plateau
The width, shape and position of the peaks obtained by CE-FA method may be affected by the
composition of running buffer and the separation voltage applied. The effects on the
plateau of clozapine peak are shown in Figure 3. Figure 3( a ) and 3( c ) show the
electropherograms of 200mmol
L-1 clozapine in rabbit plasma sample with separation voltage of -10kV and
-6kV, respectively. It is observed that resolution between the peaks of unbound clozapine
and endogenous substance decreased with an increase in applied voltage and that no plateau
of free clozapine formed at voltage of -10kV. Figure 3( b ) and 3( c ) show the
electropherograms of 200mmol
L-1 clozapine in rabbit plasma with running phosphate buffer without and with
0.5mol L-1 glycine and 1mmol L-1 EDTA, respectively. As for the
former, the plateau region of clozapine peak was significantly disturbed which might be
due to the adsorption of proteins to untreated fused silica capillary. In the latter,
however, by adding glycine containing amino group in its molecule that could reduce
protein adsorption on the capillary wall by saturating sites on the surface that otherwise
interact with proteins. The low conductivity of glycine would reduce the current and then
the Joule heat during electrophoresis, and therefore improve the band shape. On the other
hand, EDTA can effectively prevent proteins from denaturalization. The adsorption of
analytes, especially proteins, to untreated fused silica capillary is a problem in the
protein binding study, which has been overcome by addition of EDTA and glycine in the
running buffer in the present study.
3.3 Comparison of the method with ultrafiltration
Rabbit plasma and serum, human plasma were spiked with 200mmol L-1 clozapine, respectively, and analyzed in
triplicate using the present CE-FA system. Unbound clozapine concentrations in HSA
solution, plasma and serum samples measured by CE-FA method were compared with those by
ultrafiltration techniques (Table 2). The mean values of unbound clozapine concentrations
measured with CE-FA method were not significantly different from those determined by
ultrafiltration (p>0.05). This agreement indicates the reliability of the developed
CE-FA method. The precision and reproducibility of present CE-FA method under the optimum
condition are shown in Table 2. As it can be seen, the within- and between-day
precisions(RSD ) were within 3%.
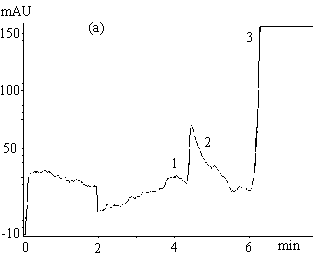
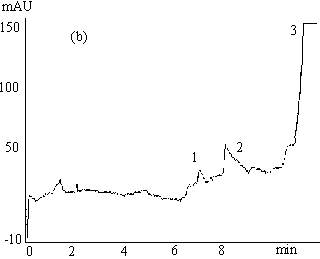
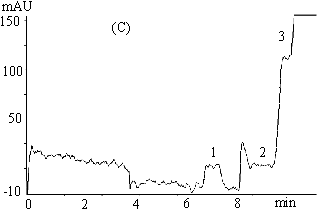
Fig.3 Electrophoregrams of 200mmol L-1 clozapine in rabbit
plasma
( a ) phosphate buffer, 67mmol L-1 (pH 7.4, containing 1mmol L-1
EDTA and 0.5mol L-1 glycine ), -10 kV;
( b ) phosphate buffer, 67mmol L-1 (pH 7.4), -6 kV;
( c ) phosphate buffer, 67mmol L-1 ( pH 7.4, containing 1mmol L-1
EDTA and 0.5mol L-1 glycine ), -6 kV.
Capillary length 0.65m (0.35m to the detector ), detection 214nm, 30s hydrostatic
injection (0.11m high ).
1 ans 3 endogenous substances, 2 free clozapine.
3.4 Investigation of protein binding of clozapine
The unbound clozapine concentrations in 550mmol L-1 HSA solution, human plasma, rabbit plasma and
serum of triple samples determined by CE-FA method were 181.8¡À2.6mmol L-1, 29.6¡À0.7mmol L-1, 31.4¡À0.4mmol L-1 and 32.2¡À0.5mmol L-1,
respectively (Table 2). The unbound clozapine concentration in 550mmol L-1 HSA solution was
significantly higher (p<0.0001) than that in human plasma (about 6 fold higher),
indicating that HSA was not the main contributor to protein binding for clozapine in human
plasma and other plasma proteins apart from albumin may be involved in the binding
process. Unbound clozapine concentration in rabbit plasma was statistically different from
that in human plasma ( p<0.05, ANOVA ) that may be resulted from a possible variation
in protein binding between animals of different species. However, the unbound clozapine
concentration measured in rabbit plasma was similar to that in rabbit serum ( p>0.05,
ANOVA ).
Table 2 Unbound clozapine in plasma and serum measured using FA-CE and
Ultrafiltration*
Sample
solution |
Concentration determined |
Concentration determined |
|
clozapine |
Within-Day |
Day-to-Day |
( mmolL-1) |
in rabbit plasma |
31.4¡À 0.4 |
31.9¡À 0.9 |
30.1¡À 0.3 |
in rabbit serum |
32.2¡À 0.5 |
32.7¡À 0.8 |
31.2¡À 0.4 |
in human plasma |
29.6¡À 0.7 |
31.1¡À 0.9 |
28.8¡À 0.3 |
in 550 mmolL-1 HSA |
181.8¡À 2.6 |
185.3¡À 4.1 |
178.6¡À 1.8 |
* Data expressed as mean ¡ÀSD (n=3).
Our initial experimental design was to determine unbound clozapine
concentrations in samples at a range of total drug concentrations (50mmol L-1, 100mmol L-1, 150mmol L-1 and 200mmol L-1). Similar trend was obtained from the results
measured in rabbit plasma, serum and human plasma at a total clozapine concentration of
150mmol L-1,
which, however, showed a lower precision. The poor concentration sensitivity of UV
detection, gives the difficulty of the determination at lower concentrations, while
further increases in clozapine concentration to > 200mmol L-1 would have little meaning for TDM and might
complicate the interaction involving multiple equilibria system.
In conclusion, a CE-FA method was developed for the investigation of
protein binding of clozapine in plasma and serum. Clozapine was well separated from
endogenous interferences and appeared as a plateau peak, the height of which measured the
concentration of unbound clozapine in sample. Factors such as sample injection time,
applied voltage and composition of running buffer affecting the separation and the
formation of the plateau peak were investigated.
The present CE-FA method may also be applicable to the binding study of
other basic drugs whose migration behavior is similar to that of clozapine with phosphate
buffer (67mmolL-1,
pH 7.4 containing 0.5mol L-1 glycine and 1mmol L-1 EDTA ) as running
buffer. A comparison study indicated that other than albumin may be involved in the
binding process of clozapine in the plasma or serum. This study demonstrated the utility
of CE-FA method in the determination of unbound drug concentration in multiple interaction
system. The advantages in terms of speed, little consumption of sample and the simplicity
of the sample pretreatment procedure would make the present method useful in TDM when the
monitoring free drug concentration is required.
REFERENCES
[1] Majcherczyk P A, Moreillon P, Decosterd L A et al. J. Pharm. Biomed. Anal., 2002, 28:
645.
[2] Shibukawa A, Ishizawa N, Kimura T et al. J. Chromatogr. B., 2002, 768: 177.
[3] Ji Z S, Li C G, Mao X A et al. Chem. Pharm. Bull., 2002, 50: 1017.
[4] Petersen C E, Ha C E, Harohalli K et al. Chem. Biol. Interact., 2000, 124: 161.
[5] Loo J A. Mass Spectrom. Rev., 1997, 16: 1.
[6] Shimura K, Kasai K I. Anal. Biochem., 1997, 251: 1.
[7] Busch M H A, Carels L B, Boelens H F M et al. J. Chromatogr. A., 1997, 777: 311.
[8]?stergaard J, Schou C, Larsen C et al. Electrophoresis, 2002, 23: 2842.
[9] Mohamed N A L, Kuroda Y, Shibukawa A et al. J. Chromatography A, 2000, 875: 447.
[10] Shibukawa A, Yoshimoto Y, Ohara T et al. J. Pharm. Sci., 1994, 83: 616.
[11] Ishihama Y, Miwa T, Asakawa N. Electrophoresis, 2002, 23: 951.
[12] Schaber G, Stevens I, Gaertner H J et al. Br. J. Clin. Pharmacol., 1998, 46: 453.
[13] Schley J, Müller-Oerlinghausen B. J. Pharm.
Pharmacol., 1986, 38: 102.