http://www.chemistrymag.org/cji/2003/05c093ne.htm |
Dec. 1, 2003 Vol.5 No.12 P.93 Copyright |
Microwave-assisted acylation of 3,4-dihydropyrimidin-2(1H)-one catalyzed by anhydrous ZnCl2
Li Baozhi, Zhang Jinsong#, Cheng Zhenhui
(College of Chemistry & Environmental
Science, Hebei University, Baoding 071002, China; #Oasis Biological Technology
CO. LTD., Jiangsu Province, Qidong 226200)
Received Sep. 15, 2003; Supported by the National Science Pre-research Foundation of Hebei University, China (No.2003Q04)
Abstract The paper reported the acylation
of 3,4-dihydropyrimidin-2 (1H)-one catalyzed by anhydrous ZnCl2 in excellent
yields under microwave irradiation. The effects of different factors on this acylation
reaction have been discussed. The experimental conditions were optimized which resulted in
a high yield 94.5% within the reaction time of 80s.
Keywords microwave irradiation; acylation; 3,4-dihydropyrimidin-2 (1H)-one
1 INTRODUCTION
In the past decade, dihydropyrimidine derivatives have exhibited important pharmacological
properties, as the integral backbone of several calcium channel blockers, antihypertensive
agent, alpha-la-antagonists, and neuropeptide Y (NPY) antagonists [1].
Recently, several isolated marine alkaloids with interesting biological activities were
also found to contain the dihydropyrimidinone-5-carboxylate core [2]. Most
notably among them are the batzelladine alkaloids, which have been found to be potent HIV
gp-120-CD4 inhibitors [3]. Consequently, synthesis of the heterocyclic nucleus
contained in such compounds has gained importance. The Biginelli reaction, first reported
more than a century ago and recently reviewed [4], involves the acid-catalyzed
cyclocondensation reaction of ethyl acetoacetate, benzaldehyde and urea. In 1934, Karl
Folkers reported that the acylated compounds were obtained after the mixture of
dihydropyrimidin-2 (1H)-one derivatives and Ac2O was refluxed for 8 hours
[5]. But the acylation of 3,4-dihydropyrimidin-2 (1H)-ones under microwave
irradiation has not been reported. Herein we report a rapid and efficient procedure for
the acylation of 3,4-dihydropyrimidin-2 (1H)-one in excellent yields under microwave
irradiation and discuss the effect of different factors. The results show microwave
irradiation can enhance this acylating reaction (Scheme 1).
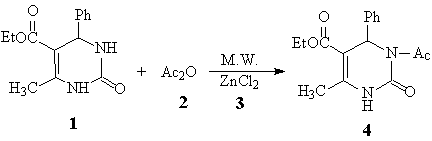
Scheme 1
2 EXPERIMENTAL
Melting points were uncorrected and were measured with micro-melting point apparatus.
IR spectra (KBr) were obtained on a Perkin-Elmer 983G spectrometer. 1H NMR
spectra were determined on a Bruker AC-80 spectrometer using CDCl3 as solvent
and tetramethylsilane (TMS) as internal reference. Mass spectra were determined on a
VG7070E spectrometer (EI, 70ev). Microwave irradiation was carried out with commercial
microwave oven (650W, 2450MHz). The products were characterized by 1H NMR
spectra and comparison of their melting point with literature values. Element analysis
data was obtained by Yanaco CHN CORDER MT-3.
General procedure. At first,
3, 4-dihydropyrimidin-2 (1H)-one had been synthesized according to the literature [4].
A mixture of 3,4-dihydropyrimidin-2 (1H)-one 0.2601g (1.00mmol), acetic hydrochloride
1.3824g (13.50mmol) and anhydrous ZnCl2 0.9548g (0.70mmol), in a cone bottle
was introduced into the microwave oven and irradiated for 30-150s (output power at 100%).
The progress of the reaction was monitored by TLC. Then, 5% hydrochloric acid (10ml) was
added to the reaction mixture. After cooling, the solution was filtrated to give a white
solid. The crude products were recrystallizated from the cooled acetic anhydride solution
in 50.3-94.5% yields.
3 RESULTS AND DISCUSSION
The paper discussed the effects of different factors on the acetylation of
3,4-dihydropyrimidin-2 (1H)-one. This acylating reaction has been investigated at various
mole ratio of 3,4-dihydropyrimidin-2 (1H)-one (1) and acetic anhydride (2)
when the power of microwave irradiation and the reaction time were kept unchanged. The
results were showed in Table 1.The yields were increased with the mole ratio of
reactant 1 and 2 up to 1:13.5 and then leveled off. When the other reaction
conditions were invariable, the effect of different microwave power on the reaction was
shown in Table 2. This may be due to the fact that the more molecules absorbs
microwave energy in short time the better the results are as microwave irradiation
intensity increases.
Table 1 The effect of the mole ratio of 3,4-dihydropyrimidin-2 (1H)-one / acetic anhydride on the reaction yield
Entry b |
Mole ratio a | Power (%) |
Reaction Time (s) |
Yield (%) |
1 |
1:20 |
100 |
80 |
85.6 |
2 |
1:15.0 |
100 |
80 |
86.9 |
3 |
1:13.5 |
100 |
80 |
94.5 |
4 |
1:10.0 |
100 |
80 |
87.7 |
5 |
1:7.0 |
100 |
80 |
82.7 |
6 |
1:5.0 |
100 |
80 |
80.5 |
a The mole ratio is 3,4-dihydropyrimidin-2
(1H)-one / acetic anhydride
b The mole ratio is 3,4-dihydropyrimidin-2 (1H)-one / anhydrous ZnCl2 is 1:0.7
Table 2 The effect of the power of microwave irradiation
Entry |
Power (%) |
Mole ratio c | Reaction Time (s) |
Yield (%) |
1 |
100 |
1:13.5:0.7 |
80 |
94.5 |
2 |
70 |
1:13.5:0.7 |
80 |
84.5 |
3 |
50 |
1:13.5:0.7 |
80 |
72.4 |
4 |
30 |
1:13.5:0.7 |
80 |
50.3 |
c The mole ratio is 3,4-dihydropyrimidin-2 (1H)-one / acetic anhydride / anhydrous ZnCl2
When the other reaction conditions were invariable, the effect of different reaction time on the acylating reaction was shown in Table 3. As summarized in Table 3, the yields were increased with the longer reaction time before 80s, after it the yields were reduced because of the increase of side reaction. In order to prevent from producing the emergence of boiling phenomenon, the reflux devices were installed while reacting in the microwave oven. When anhydrous AlCl3 was substituted for anhydrous ZnCl2 as catalyst, the satisfied results were obtained (Table 5). Therefore, it may be the fact that Lewis acids have good catalysis to the acylating reaction.
The yield was up to 94.5% at first, the melt point agrees with document value (Found:176-177ºC; Reported [5]: 175.5-177ºC). Secondly, The structure of the product is confirmed through element analysis (Calculated: C-63.58, H-5.96, N-9.27; Found: C-63.41; H-6.00, N-9.18) and H1NMR, IR, consistent with the literature report [7]. Finally, The reaction time was largely shortened compared with reported 8 hours under conventional heating [5].Table 3 The effect of the reaction time of the acylation of 3,4-dihydropyrimidin-2 (1H)-one
Entry |
Mole ratio c | Power (%) |
Reaction Time (s) |
Yield (%) |
1 |
1:13.5:0.7 |
100 |
30 |
88.4 |
2 |
1:13.5:0.7 |
100 |
60 |
90.7 |
3 |
1:13.5:0.7 |
100 |
70 |
92.5 |
4 |
1:13.5:0.7 |
100 |
80 |
94.5 |
5 |
1:13.5:0.7 |
100 |
90 |
93.4 |
6 |
1:13.5:0.7 |
100 |
120 |
87.1 |
7 |
1:13.5:0.7 |
100 |
150 |
75.5 |
c The mole ratio is 3,4-dihydropyrimidin-2 (1H)-one / acetic anhydride / anhydrous ZnCl2
Table 4 The effect of the quantity of Catalyst (anhydrous ZnCl2) used in the reaction
Entry |
Mole ratio a | Power (%) |
Reaction Time (s) |
Yield (%) |
1 |
1:1.00 |
100 |
80 |
88.4 |
2 |
1:0.70 |
100 |
80 |
94.5 |
3 |
1:0.50 |
100 |
80 |
89.4 |
4 |
1:0.25 |
100 |
80 |
87.1 |
5 |
1:0.10 |
100 |
80 |
75.5 |
a The mole ratio is 3,4-dihydropyrimidin-2 (1H)-one / anhydrous ZnCl2
Table 5 The effect of the quantity of Catalyst (anhydrous AlCl3) used in the reaction
Entry |
Mole ratio d | Power (%) |
Reaction Time (s) |
Yield (%) |
1 |
1:1.00 |
100 |
80 |
87.5 |
2 |
1:0.70 |
100 |
80 |
90.4 |
3 |
1:0.50 |
100 |
80 |
87.2 |
4 |
1:0.25 |
100 |
80 |
84.3 |
5 |
1:0.10 |
100 |
80 |
65.5 |
d The mole ratio is 3,4-dihydropyrimidin-2 (1H)-one / anhydrous AlCl3
4 CONCLUSIONS
It was concluded that the best synthetic
conditions of the 3,5-diacetyl-6-methyl-4-phenyl-3,4-dihydropyrimidin-2 (1H)-one (4) were the mole ratio of 3,4-dihydropyrimidin-2 (1H)-one
and acetic anhydride is 1:13.5, 3,4-dihydropyrimidin-2(1H)-one and anhydrous ZnCl2
is 1:0.7, the power of microwave irradiation was 100%(2450MHz), reaction time was 80s, and
the yield of 4 was up to 94.7%. In
conclusion, a rapid and efficient method for the preparation of
3,5-diacetyl-6-methyl-4-phenyl-3, 4-dihydropyrimidin-2 (1H)-one has been provided, which
has the characteristics such as its simplicity in operation, high yields, short reaction
time and low cost. In addition, pollution can be further reduced.
REFERENCES
[1] Rovnyak G C, Kimball S D, Beyer B, et al. J. Med.Chem. 1995, 38: 119.
[2] Snider B B, Shi Z. J. Org. Chem. 1993, 58:3828.
[3] Snider B B, Chen J, Patil A D, et al. Tetrahedron Lett. 1996, 37: 6977.
[4] Kappe C O. Tetrahedron. 1993, 49: 6937.
[5] Folkers K, Johnson J B. J. Am. Chem. Soc.1934, 56:1374.
[6] Lu J, Yang B Q, Bai Y J, Ma H R. Chin. J. Org. Chem. 2001, 21: 640.
[7] Christian O K, Peter R. J. Heterocyclic Chem. 1989, 26:55.