http://www.chemistrymag.org/cji/2003/05c092pe.htm |
Dec. 1, 2003 Vol.5 No.12 P.92 Copyright |
The crystal structure of 3-[5-methyl-1-(4-methylphenyl)-1,2,3-triazol -4-yl]-6-(4-methylphenyl)-s-triazolo[3,4-b]-1,3,4-thiadiazole
Zhuang Shanxue2, Zhu Anxiong4, Cai Lixiang3, Quan Bin2, Dong Hengshan1(1National Laboratory of Applied Organic Chemistry, Institute of Organic Chemistry, College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu 730000; 2Department of Chemistry, Guansu Education College. Lanzhou, Gansu 730000; 3Instrumental Analysis & Research Center, College of Chemistry and Chemical Engineering, Lanzhou University; 4Yongchan No. 4 Middle School, Gansu) Received Sep. 15, 2003; Supported by National Natural Science Foundation of China (No.20021001) and Lanzhou University NLAOC.
Abstract A novel compound 3-[5-methyl-1-(4-methylphenyl)-1,2,3-triazol-4-yl]-6 -(4-methylphenyl)-s -triazolo[3,4-b]-1,3,4- thiadiazole was synthesized from p-methylaniline to the mixed triazoles derivative 5 then reacted with p-methylbenzoic acid. The title end product 6 was investigated with X-ray crystallographic, NMR, MS and IR techniques. Compound 6, C20H17N7S, Mr=387.47, crystallized in the monoclinic space group P21/c with unit cell parameters a=6.640(3), b=17.999(4), c=18.004(8)Å, a=90.07(3), b=91.85(4), g=90.01(3)º.
Keywords Crystal structure; 1,2,3-triazole; s-triazolo[3,4-b]-1,3,4-thiadiazole.
It is reported that the 1,3,4-triazole
nucleus possesses fungicidal[1], insecticidal[2], antimicrobial[3],
bactericidal[4] properties and the 1,2,3-triazole nucleus possesses
antibacterial[5], antifungal[6], antiviral[7],
anti-inflammatory and analgesic[8] properties. Recently some new 1,3,4-triazole
derivatives have been reported as possible anticonvulsants[9] and plant growth
regulators[10] and the 1,2,3-triazole derivatives have been reported that
inhibits tumor proliferation, invasion, and metastasis[11]. Likewise the
1,3,4-thiadiazole nucleus which incorporates an N-C-S linkage exhibits a large number of
biological activities[12]. The fused 1,3,4-triazolo[3,4-b]-1,3,4-thiadiazoles
derivatives show various biological effects, such as antifungal[13],
antibacterial, hypotensive and CNS depressant activities[14]. A
triazolo-thiadiazole system may be viewed as a cyclic analog of two very important
components-thiosemicarbazide and biguanide, which often display diverse biological
activities. Therefore, it was planned to investigate a system, which combines these three
biologic components in a ring to give a compact system for screening their biologic
activities.
We have reported the crystal structure of
2-(3-bromoanilino)-5-[5-amino-1-(4- chlorophenyl)-1,2,3-triazole-4-yl]-1,3,4-thiodiazole
and their derivatives.[15] Recently, we obtained a novel compound 3-[5-methyl-1-(4-methylphenyl)-1,2,3-triazol-4-yl]-6-(4-
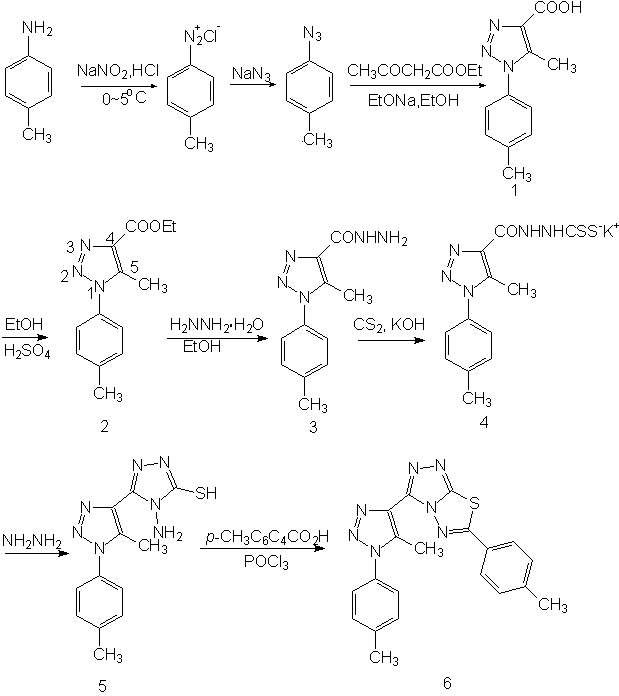
methylphenyl)-s-triazolo[3,4-b]-1,3,4-thiadiazole. The route of syntheses is shown
in scheme 1.
Melting points were determined on a Kofler melting point apparatus and are uncorrected. The mass spectrum was performed on a HP-5988A spectrometer (EI at 70 eV). IR spectra were obtained in KBr discs using a Nicolet 170SX FT-IR spectrometer. 1H NMR spectra were recorded at room temperature at 80.1MHz on a Bruker FT-AC 80 instrument. Elemental analyses were carried out on a Yanaco CHN Corder MT-3 analyzer.
Phosphorus oxychloride was redistilled (bp 105ºC). 5-Methyl-1-(4-methylphenyl)-1,2,3-triazol -4-carboxylic acid 1 was prepared following the methods in the literature.[16]
The preparation of ethyl 5-methyl-1-(4-methylphenyl)-1,2,3-triazol-4-carboxylate 2. In a 150mL round bottomed flask was placed a mixture of 21.7g(0.10 mol) of 1, 46g (59mL, 1.0 mol) of absolute ethanol and 6mL of concentrated sulfuric acid. The mixture was refluxed gently for 10 h, cooled to room temperature, was refrigerated for 10-12 h. A white solid was obtained and filtered, washed with absolute ethanol, recrystallized by absolute ethanol. The compound 2 was a white crystalline solid, m.p. 130-132ºC, yield 19.7g (80.6%).
Scheme 1
5-Methyl-1-(4-methylphenyl)-1,2,3-triazol-4-carbonylhydrazine 3 was prepared from 2 following the method reported in the literature.[17]
A mixture of 0.1 mol of 2 and 0.15 mol of hydrazine hydrate(85%) in 200mL of ethanol was refluxed for six hours. The ethanol, water, and excess hydrazine hydrate were removed in vacuo, and the residual solid was recrystallized. It gives 21g (91% yield) of product 3, m.p. 177-178ºC.
1-Amino-2-mercapto-5-[5-methyl-1-(4-methylphenyl)-1,2,3-triazol-4-yl]-1,3,4-triazole 5 was prepared from 3 via 4 following the method reported in the literature.[18]
Carbon disulfide (0.14 mol) was added dropwise to an ice-cold solution of potassium hydroxide (0.15 mol) and carbonylhydrazine 3 (0.09 mol) in 150mL absolute ethanol. The mixture was stirred at room temperature for 14 h. Dry ether (200mL) was then added and the separated solid was filtered and washed with ether (2¡Á50mL). The product 4 obtained in nearly quantitative yield was employed in the next reaction without further purification.
A suspension of 4 (about 0.08 mol) and hydrazine hydrate 85% (0.16 mol) in 50mL of water was refluxed while stirring for 4 h. The color of the reaction mixture changed to green, hydrogen sulfide evolved, and a homogeneous solution resulted. On dilution with 850mL of cold water and acidification with concentrated HCl, a white solid precipitated. The product was filtered, washed with water and recrystallized from ethanol to give 18g (71% yield) white flakes of 5, m.p. 189-190ºC.
Preparation of 3-[5-methyl-1-(4-methylphenyl)-1,2,3-triazol-4-yl]-6-aryl-s-triazolo[3,4-b]-1,3,4- thiadiazole 6.[19]
A mixture of 5 (1 mmol), p-MeC6H4COOH(1 mmol), and POCl3 (5mL) was heated under reflux for 6 h. The reaction mixture was cooled. After removal of excess POCl3, the solid residue was poured into ice water, made alkaline by adding potassium hydroxide and the resulting solid was filtered. The solid was recrystallized from ethanol to give the title compound. The solid was separated by chromatographic column (silica gel, eluant: ethyl acetate:petroleum ether 1:1). Compound 6. Yield 27%, mp 209-210ºC, IR umax: 3370, 3295, 3229, 3177(m, -N-H), 3022(Ar-H), 2918(w, CH2 or CH3), 1611, 1517, 1460(s, Ar), 1261(C=N-N), 951(w, -N-N=N-)cm-1. 1H NMR dH: 7.43(s, 4H, Ar), 7.28-7.91(q, 4H, Ar), 2.49(s, 3H, CH3), 2.74(s, 3H, Ar1CH3), 2.46(s, 3H, Ar2CH3)ppm. Analysis for C20H17N7S, Mr=387.47, calculated C 62.00, H 4.42, N 25.31, S 8.27; found C 62.22, H 4.35, N 25.12 S 8.31. MS M/z: 387(

Fig. 1 ORTEP drawing of the title compound showing the atom numbering scheme
The purified product was
dissolved in ethyl acetate: petroleum ether: ethanol solution. The crystal was obtained
after 7 d by evaporation of the solution.
A single crystal was selected and mounted on the tip of a glass fiber.
Preliminary examination and data collection was performed with MoK
The structure of the title compound is shown in Fig. 1. The unit cell packing of the title compound is shown in Fig. 2. The fractional coordinates and mean temperature factors with estimated standard deviations for non-hydrogen atoms are listed in Table 2 and selected bond lengths are given in Table 3, selected bond angles are given in Table 4. The geometric calculations were performed using the program SHELX-97.
Table 1 Crystal data and summary of data collection and structure refinement
Compound |
C20H17N7S | Dcalc, g cm-3 |
1.197 |
Color/Shape |
colorless/rhombus |
F(000) |
808 |
Formula weight |
387.47 |
Absorption coefficient m m-1 |
0.169 |
Temperature, ºC |
20(293K) |
Index ranges |
0¡Üh¡Ü6;0¡Ük¡Ü17; |
Crystal system |
Monoclinic |
Standard reflections |
3778 |
Space group |
P21/c |
Reflections measured |
2060 |
Cell constants |
Reflection observed |
[1>2 s(I)] 255(Rint=0.0320) |
|
a, Å |
6.640(3) |
Maximum value of q(¡ã) |
25.02 |
b, Å |
17.999(4) |
Computing |
Data collection CAD4 |
c, Å |
18.004(8) |
Cell refinement CAD4 |
|
| a, deg. | 90.07(3) |
Data reduction PCSDP |
|
| b, deg. | 91.85(4) |
Structure solution SHELXS-97 |
|
| g, deg. | 90.01(3) |
Goodness-of-fit on F2 |
1.068 |
Volume, Å3 |
2150.6(14) |
Final R indices |
R1=0.0320, |
Formula units/units cell |
4 |
Largest diff. Peak and hole |
0.051 and |



Fig. 2 The unit cell packing of the title compound
Table 2 The fractional coordinates and mean temperature factors with estimated standard deviations for non-hydrogen atoms
x |
y |
z |
Ueq | C7 |
1.0707(18) |
0.7784(7) |
0.4874(7) |
0.056(4) |
|
S1 |
1.7820(5) |
0.4579(2) |
0.9269(2) |
0.074(4) |
C8 |
1.0216(17) |
0.6672(10) |
0.7524(7) |
0.071(5) |
N1 |
1.2380(13) |
0.6586(5) |
0.6445(5) |
0.045(4) |
C9 |
1.204(2) |
0.6412(7) |
0.7153(8) |
0.057(4) |
N2 |
1.4216(17) |
0.6334(10) |
0.6248(7) |
0.073(5) |
C10 |
1.3749(18) |
0.6077(9) |
0.7389(8) |
0.057(5) |
N3 |
1.5055(15) |
0.6033(8) |
0.6830(7) |
0.067(4) |
C11 |
1.423(2) |
0.5748(7) |
0.8113(7) |
0.051(4) |
N4 |
1.5881(15) |
0.5317(5) |
0.8294(6) |
0.047(4) |
C12 |
1.573(2) |
0.5136(7) |
0.9008(8) |
0.060(5) |
N5 |
1.7460(17) |
0.5027(6) |
0.7893(5) |
0.057(4) |
C13 |
1.8531(19) |
0.4668(7) |
0.8345(7) |
0.060(5) |
N6 |
1.3236(17) |
0.5813(6) |
0.8737(5) |
0.055(4) |
C14 |
2.040(2) |
0.4252(9) |
0.8089(8) |
0.062(5) |
N7 |
1.426(2) |
0.5438(7) |
0.9294(8) |
0.074(5) |
C15 |
2.098(2) |
0.4314(9) |
0.7366(8) |
0.073(6) |
C1 |
0.740(3) |
0.7982(16) |
0.4192(14) |
0.103(7) |
C16 |
2.268(5) |
0.4009(17) |
0.7160(16) |
0.106(7) |
C2 |
0.869(2) |
0.7573(8) |
0.4773(8) |
0.061(5) |
C17 |
2.388(2) |
0.3538(9) |
0.7602(11) |
0.075(5) |
C3 |
0.7951(15) |
0.7049(9) |
0.5235(7) |
0.062(5) |
C18 |
2.330(3) |
0.349(2) |
0.8340(19) |
0.111(11) |
C4 |
0.910(2) |
0.6713(8) |
0.5788(7) |
0.060(4) |
C19 |
2.158(3) |
0.3858(11) |
0.8567(17) |
0.092(7) |
C5 |
1.1118(14) |
0.6934(8) |
0.5884(6) |
0.049(4) |
C20 |
2.569(3) |
0.3139(14) |
0.7358(17) |
0.112(8) |
C6 |
1.1941(15) |
0.7483(9) |
0.5429(6) |
0.058(5) |
Table 3. Selected bond lengths(Å)
S1 |
C13 |
1.752(14) |
N6 |
C11 |
1.33(2) |
C9 |
C10 |
1.34(3) |
S1 |
C12 |
1.763(14) |
N6 |
N7 |
1.37(2) |
C10 |
C11 |
1.46(3) |
N1 |
C9 |
1.339(19) |
N7 |
C12 |
1.24(3) |
C13 |
C14 |
1.53(2) |
N1 |
N2 |
1.358(17) |
C1 |
C2 |
1.52(3) |
C14 |
C19 |
1.35(3) |
N1 |
C5 |
1.436(18) |
C2 |
C3 |
1.36(2) |
C14 |
C15 |
1.37(2) |
N2 |
N3 |
1.29(2) |
C2 |
C7 |
1.40(2) |
C15 |
C16 |
1.32(4) |
N3 |
C10 |
1.35(2) |
C3 |
C4 |
1.38(2) |
C16 |
C17 |
1.39(4) |
N4 |
C12 |
1.33(2) |
C4 |
C5 |
1.400(19) |
C17 |
C18 |
1.40(4) |
N4 |
C11 |
1.37(2) |
C5 |
C6 |
1.41(2) |
C17 |
C20 |
1.48(3) |
N4 |
N5 |
1.394(18) |
C6 |
C7 |
1.38(2) |
C18 |
C19 |
1.39(5) |
N5 |
C13 |
1.24(2) |
C8 |
C9 |
1.48(2) |
Table 4. Selected bond angles(¡ã)
C13 |
S1 |
C12 |
85.9(7) |
C3 |
C4 |
C5 |
118.0(12) |
N7 |
C12 |
S1 |
139.4(12) |
C9 |
N1 |
N2 |
110.4(12) |
C6 |
C5 |
C4 |
121.1(11) |
N4 |
C12 |
S1 |
108.3(11) |
C9 |
N1 |
C5 |
131.4(10) |
C6 |
C5 |
N1 |
119.3(9) |
N5 |
C13 |
C14 |
120.6(12) |
N2 |
N1 |
C5 |
118.1(10) |
C4 |
C5 |
N1 |
119.7(11) |
N5 |
C13 |
S1 |
120.2(12) |
N3 |
N2 |
N1 |
107.3(11) |
C7 |
C6 |
C5 |
117.7(9) |
C14 |
C13 |
S1 |
119.1(9) |
N2 |
N3 |
C10 |
108.0(11) |
C6 |
C7 |
C2 |
122.0(12) |
C19 |
C14 |
C15 |
118.4(17) |
C12 |
N4 |
C11 |
106.5(12) |
C10 |
C9 |
N1 |
104.0(13) |
C19 |
C14 |
C13 |
121.7(18) |
C12 |
N4 |
N5 |
119.2(11) |
C10 |
C9 |
C8 |
134.2(13) |
C15 |
C14 |
C13 |
119.8(12) |
C11 |
N4 |
N5 |
134.1(10) |
N1 |
C9 |
C8 |
121.3(12) |
C16 |
C15 |
C14 |
119.8(16) |
C13 |
N5 |
N4 |
106.2(10) |
C9 |
C10 |
N3 |
110.3(14) |
C15 |
C16 |
C17 |
125(2) |
C11 |
N6 |
N7 |
109.0(13) |
C9 |
C10 |
C11 |
128.7(13) |
C16 |
C17 |
C18 |
114(2) |
C12 |
N7 |
N6 |
106.9(13) |
N3 |
C10 |
C11 |
120.9(12) |
C16 |
C17 |
C20 |
125(2) |
C3 |
C2 |
C7 |
118.1(13) |
N6 |
C11 |
N4 |
105.4(11) |
C18 |
C17 |
C20 |
120(2) |
C3 |
C2 |
C1 |
123.4(16) |
N6 |
C11 |
C10 |
128.3(14) |
C17 |
C18 |
C19 |
120.3(19) |
C7 |
C2 |
C1 |
118.3(15) |
N4 |
C11 |
C10 |
126.4(11) |
C14 |
C19 |
C18 |
122(3) |
C2 |
C3 |
C4 |
123.0(11) |
N7 |
C12 |
N4 |
112.1(13) |
The structure of the title compound is shown in Fig. 1. In recent years the synthesis and characteristics of s-triazolo[3,4-b]-1,3,4-thiadiazoles have been investigated.[21] These heterocyclic compounds contain 1,2,4-triazole and 1,3,4-thiadiazole rings condensed through a C-N bond. The substituted groups at the 3 or 6 positions can conjugate with the heterocyclic nucleus thus giving it new characteristics. In a continuation of our earlier studies,[15] we now report the crystal structure of 3-[5-methyl-1-(4-methylphenyl)-1,2,3-triazol-4-yl]-6-(4-methylphenyl)-s-triazolo[3,4-b]-1,3,4-thiadiazole 6.
The central ring system in the present compound does not differ significantly from those in the structures of the two other substituted s-triazolo[3,4-b]-1, 3, 4-thiadiazole compounds already determined, the 3-[2-aminophenyl]-6-tolyl derivative. The bond lengths indicate a degree of delocalization around the ring system with the C=N bonds ranging from 1.24(3) to 1.436(18)Å and the N-N bond lengths ranging from 1.29(2) to 1.394(18)Å.
The 1,2,3-triazole ring system is planar. The bond lengths are shortened [N1-N2(N-N 1.41Å[20]) 1.358(17)Å], lengthened [N2-N3(N=N 1.23Å) 1.29 (2)Å], which are in agreement with the values reported for triazole, N-N 1.352Å by Fenean-Dupont and Huang.[21] The bond lengths C10-N3 1.35(2)Å and C9-N1 1.339(19)Å are between the bond lengths of C=N(1.27Å) and C-N(1.45Å). REFERENCES
[1] Hausach C, Sachse B, Buerstell H. Ger Offen, 2,826,760 (Chem Abstr: 1980, 92: 181200h).
[2] Tanka G. Japan Kokai, 7495,973 (Chem Abstr: 1975, 82: 156320h).
[3] Griffin A D, Sally K. EP 119474 (Chem Abstr: 1986, 106: 98120).
[4] Van Reet G, Heeres J. US Pat, 4,160,838 (Chem Abstr: 1979, 91: 753612)
[5] Zhang Z Y, Liu Y, Yang S Y. Pharmaceutica Simica, 1991, 26 (11): 809.
[6] Abdou N A, Soliman I N, Sier Abou A.H. Bull Facpharm (Cairo Univ.), 1991, 28 (6): 29.
[7] Srivatava J, Swarup S, Saxena V K. J. Indian Chem. Soc., 1991, 68 (2): 103.
[8] Cooper K, Steele J. EP 329357 (Chem Abstr: 1990, 112: 76957).
[10] Husain M I, Amir M. J. Indian Chem. Soc., 1986, 63: 317.
[11] Raymond E, Raymond S, Alan G S. GB 2,175,301 (Chem Abstr: 1987, 107: 134310).
[12] Kohn E C, Liotta L A. US 637145 (Chem Abstr: 1991, 115: 248099).
[13] Omar A Mohsen,E, Aboul W, Omainma M. J. Heterocycl. Chem., 1986, 23: 1339.
[14] Bano Q, Tiwari N, Giyi S,et al. Indian J. Chem., 1992, 31B: 714.
[15] Zhang Z Y, Dong H S, Zhu Y. Acta Chimica Since, 1996, 54: 1054.
Zhang Z Y, Dong H S, Guan Z W, Yang S Y. Chem. Research Chin. Univ., 1997, 13(1): 27.
Zhang Z Y, Dong H S, Yang S Y. Organic Chemistry, 1996, 16: 430; 1998, 18: 253.
[16] Khaclem H E L, Mansour H A R, Meshreki M H. J. Chem. Soc(c)., 1968, 1329.
[17] Yale H I, Losee K, Martin J, Hervy H, Pervy F M, Bernstain J. J. Amer. Chem. Soc., 1953, 75: 1933.
[18] El-Barbary, Fahmy M, El-Badawi M, El-Bremaly, El-Brollosi N R. Revue Roumaine de Chimie,1991, 36 (4-7): 619.
[19] Dong H Sh, Wei K, Wang Q L, Quan B. Synth. Commun., 2001, 31 (1): 81-88.
[20] Zhang Z Y, Guan Z W, Chen Xin. Heterocyclic Chemistry (Zahuan Huaxue), Lanzhou Daxue Chubanshe,1992: 15.
[20] Feneau-Dupont J, Deelereq J P, Vanderstede E, L'abbe' G. Bull. Soc. Chim. Belg., 1989, 98: 415
[21] Huang Z T, Wang M X. Chin. Chem. Lett., 1990, 1: 5.
¡¡
¡¡