http://www.chemistrymag.org/cji/2003/05c097pe.htm |
Dec. 1, 2003 Vol.5 No.12 P.97 Copyright |
Quenching electrochemiluminescential analysis of pico-molar level glutathione
Tu Yifeng,
Qi Yingying, Wu Ying, Di Junwei
(College of Chemistry and Chemical Engineering, Suzhou University, Suzhou,
215006, China)
Abstract Based
on the study of highly sensitized electrochemiluminescence (ECL) of luminol by iodide, in
neutral medium, because of the production of free radicals, the authors have developed a
sensitive quenching ECL method to determine the reductive glutathione(GSH) due to its
elimination for the free radicals. The detect limit of this method has decreased to
pico-molar level. It is more sensitive than the methods ever reported .
Keywords Electrochemiluminescence; Luminol;
Quenching; Glutathione
1. INTRODUCTION
The electrochemiluminescence(ECL)[1] is in prospect to be used in trace
analysis, immunoassay, biochemical analysis and some other fields[2,3]. In
general, the ECL behaviour of luminol in alkaline medium is superior in intensity to that
of in neutral medium[4]. But for the object to determine the bioactive
molecules, the neutral medium would be more suitable for keeping their original
properties. The authors have already studied the ECL behaviour of luminol in neutral
medium[5]. Although it revealed that the ECL of luminol in neutral condition
was weaker than that in alkaline medium, it has been indicated that some redox species
such as oxygen, hydrogen peroxide and iodide can enhance the luminous intensity of luminol
in neutral medium on a large scale. It provides an excellent basis to determine the
glutathione(GSH)[6] by quenching effect for iodide enhanced ECL and furthermore
to develop a sensitive analytical method of glutathione at pico-molar level of
concentration.
The glutathione widely distributes in natural world. It is an important
bioactive peptide for eliminating the free radicals, detoxicating, postponing the aging
and relieving the fatigue. It is also the participant of many enzymatic reactions as the
role of coenzyme to transfer the electrons. The studies on its determination and the
reaction mechanism have attracted great attentions. The improvement on its sensitive
determination is especially expected. The ECL quenching method for determination of
glutathione, which will be described in this paper, can detects it with a limit
concentration at pico-molar level. It is the most sensitive one in the previously reported
methods. The probable mechanism of this method is also discussed.
2. EXPERIMENTS
2.1 Instruments and chemicals
A BAS-100A electrochemical analyzer (Bioanalysis System Inc., USA) was used as a
potentiostat with a XFD-8C ultralow frequency signal generator (Ningbo Radio Factory,
P.R.China) as the outer pulse signal source. A PMT-II faint light meter, made by the
authors, was used to detect the luminous intensity of ECL. The scheme of whole
installation is presented in our previous paper[7].
A three-electrode cell was used in experiments. The working electrode
and the auxiliary electrode were made of platinum. The working electrode was firstly
polished with Al2O3 powder and then washed with 0.1mol/L NaOH and
then water in ultrasonic tank. A silver wire worked as a reference electrode. The glass
cell was prepared in advance. It was covered with a silver film that formed in a silver
mirror reaction merely reserved a hole to keep transparence for light. And then it was
covered with the black paint. The hole was situated in the center of the window of
photoelectric multiplier (PMT). The cell and the PMT were sealed in a black box.
Luminol was purchased from Fluka. The reductive glutathione is the
product of Shanghai Chemicals Cooperation. The solution of reductive glutathione was
prepared by dissolving it in deoxygenated water. Other chemicals are all analytical
reagents and water used in all experiments is the sub-boiling distillate water, prepared
in a quartz apparatus.
2.2 The method of determination
The determination was carried out in the phosphate buffer solution (pH 6.7) which
containing 4.0กม10-4 mol/L of luminol and 4.0กม10-5 mol/L of iodide.
The quenched ECL intensity was recorded after certain volume of glutathione solution had
been injected into this solution.
3. RESULTS AND DISCUSSIONS
3.1 The optimized conditions for determination of glutathione
The different kinds of buffer solution could not only result in the difference of luminous
intensity, but also the sensitization efficiency of sensitizers. So it must be selected
carefully. From the results of experiments, the phosphate buffer (pH 6.7) was the best one
for iodide enhancing ECL of luminol and for occurrence of the quenching effect from
glutathione. The aforementioned concentrations of luminol and iodide contained in buffer
solution were selected for providing a proper luminous intensity. They were profitable for
detecting and recording the ECL background and the decreasing after the glutathione was
injected into the solution.
In a single pulse period, the potential of pulse signal was set at the
constantly lower voltage of 0.0V for 5s, and then it would raise to the higher voltage of
1.5V for 1s, which resulted in the generating of ECL during this stage. The longer period
of low potential was sufficient for achieving the equilibrium of reactants by diffusion.
The ECL intensity could be stable under this condition.
3.2 The ECL response of reductive glutathione
Under aforementioned conditions, this method is sensitive for the determination of
glutathione. When the glutathione was injected into the solution, the ECL luminous
intensity remarkably decreased immediately. Fig. 1 shows the ECL curves of luminol, after
sensitization from iodide and then quenched by glutathione.
The quenched ECL intensity linearly responded to the concentration of
reductive glutathione within a wide range. It leaped over eight orders of magnitude from
10-5 mol/L to 10-13 mol/L with a detecting limit of 3.38กม10-13mol/L.
The attached equation in Fig. 1 is the calibration equation of glutathione on this range
and Tab.1 shows the calibration equations of glutathione in several orders of magnitude
and the linear regression coefficients.
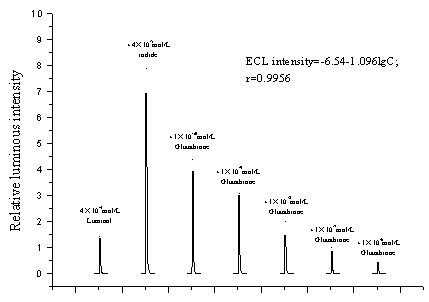
Fig.1 The ECL curves of luminol,
sensitized by iodide and quenched by glutathione
กก
Table 1 The calibration equations of glutathione in several orders of magnitude and the linear regression coefficients
Concentration range |
Calibration equation |
Regression coefficient |
10-11 mol/L |
Y=18.5-1.54C |
-0.999 |
10-12 mol/L |
Y=35.24-2.56C |
-0.993 |
10-13 mol/L |
Y=23.98-1.17C |
-0.993 |
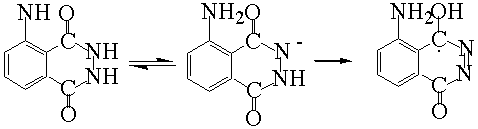
3.3 The mechanism of quenching effect
of glutathione for ECL
The iodide enhanced ECL of luminol could be described as a procedure of producing and
reacting of free radicals.
I-![]() I·
I·
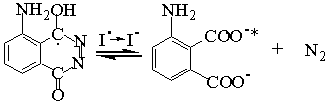
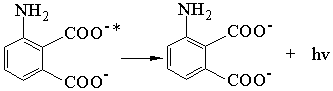
GSH + R· ![]() GS·
+ RH (The R· standing for all of free radicals)
GS·
+ RH (The R· standing for all of free radicals)
There are two experimental
evidences to prove that the iodides were oxidized to form free radicals. Firstly, the ECL
intensity of luminol-I-1 solution was obviously come under the influence of
amplitude of rectangle pulse (See Fig. 2).
It presented a regular pattern that the ECL intensity would
quasi-linearly increase within the potential of 1.5V but never emit light if the potential
was higher than 1.5V. This phenomenon could be understood as that different products were
produced under different potential. From the studies of luminol's ECL in neutral medium[8],
it was known that the luminol could emit the light after the first-stage oxidation at the
potential of 0.75-0.9V and be followed by other chemical oxidation especially from some
free radicals. So, it could be considered that the pulse potential provided the energy to
oxidize the iodide to free radical but not the iodine. It is reasonable that the adaptive
potential could result in the production of free radicals, but higher potential might lead
to quickly annihilation of free radicals of iodide to produce the iodine due to high
concentration, it could not go on to aid the luminescence of luminol's first-stage
oxidized product in this condition.
Another hand, it had shown a special phenomenon that the influence of
iodide to the ECL intensity would be different within different concentration ranges. The
ECL intensity will trend to decrease if there is higher concentration of iodide other than
it could sensitize the ECL at lower concentrations (See Fig.3).
The believable explanation is that the annihilation of iodide free
radicals might be in greater degree at higher concentrations of iodide because of the
higher production of free radicals.

Fig. 2 The response of ECL upon the
amplitude of pulse

Fig. 3 The variation of ECL
intensity with the concentration of iodide
4. CONCLUSION
The experimental results have indicated that the glutathione is an excellent eliminator
for free radicals. It results in the sensitive quenching effect for that of the iodide
sensitized electrochemiluminescence of luminol in neutral medium. It is hopeful for
developing a method for the analysis of glutathione.
REFERENCES
[1] Knight A W, Greenway G M. Analyst, 1994, 119: 879.
[2] Soper S A, Warner I M, McGown B. Anal. Chem., 1998, 70: 477.
[3] Jamelson F, Sanchez R I, Dong L W et al. Anal. Chem., 1996, 68: 1298.
[4] Kulmala S, Ala-Kleme T, Kulmala A et al. Anal. Chem., 1998, 70: 1112.
[5] Tu Y F, Huang B Q, Guo W Y et al. Chinese J. Anal. Chem. (Fenxi Huaxue), 2002, 30 (6):
729.
[6] Chen S, Ding H Q. Progress in Physiological Sciences (Shengli Kexue Jinzhan), 2002, 33
(1): 85.
[7] Tu Y F, Guo W Y, Huang B Q et al. Chinese J. Spectroscopy Laboratory (Guangpu
Shiyanshi). 2001, 18 (2): 185.
[8] Huang B Q, Guo W Y, Xu Y et al. Spectroscopy & Spec. Anal. (Guangpuxue Yu Guangpu
Fenxi), 2003, 23 (5): 849.