http://www.chemistrymag.org/cji/2003/05c096pe.htm |
Dec. 1, 2003 Vol.5 No.12 P.96 Copyright |
(College of Chemistry & Environmental Science, Hebei University, Baoding 071002; #Institute of Chemistry, Chinese Academy of Sciences, Beijing 100080, China)
Abstract The kinetic and mechanistic
features of potassium ditelluratoargentate (III) (DTA) initiated aqueous polymerization of
acrylamide (AAM) have been investigated in an alkaline medium. The polymerization
behaviors as a function of [AAM], [DTA], pH as well as temperature, have been studied. The
overall rate of polymerization has been determined from gravimetry. The rate has been
found to bear 1.68 and 0.76 dependence on [AAM] and [DTA], respectively. The overall
activation energy of AAM polymerization is calculated as 33.6kJ/mol. Based on the FTIR and
1H NMR spectra analyses, a tentative initiation mechanism involving a two-step
single-electron-transfer process is proposed.
Keywords potassium ditelluratoargentate (III); redox initiation; acrylamide;
aqueous polymerization
1. INTRODUCTION
Recently, studies on the transition metals in a higher oxidation state have been the
most active area. Investigations on them such as Cu (III), Ag (III), Ni (IV), Ce (
In our previous study, potassium diperiodatoargentate (III) (DPA) coupled with reductant showed as an effective initiator in the homopolymerization of acrylamide [13] and acrylonitrile [14], as well as in the graft copolymerization of vinyl monomer onto macromolecule such as methyl acrylate on starch [15], casein [16], and nylon [17-18]. A two-step single-electron-transfer mechanism in the reduction process of DPA is well established to explain the formation of radicals and the initiation. For further verifying that the mechanism holds true for DTA and finding a new redox initiation system, it is valuable for us to study the application of DTA in polymerization reaction. In the present paper, the kinetics of aqueous polymerization of acrylamide initiated by DTA is reported and a plausible initiation mechanism based on a two-step single-electron-transfer process is proposed.
2. EXPERIMENTAL
2.1 Materials
All the chemicals were A.R. grade and were used without further
purification. Water was doubly distilled over alkaline permanganate. The same procedure as
described earlier [19] was used to prepare the stock solution of DTA, which was
preserved in a refrigerator. The concentration of DTA was standardized
spectrophotometrically at its absorption maximum i.e. l=350nm where the oxidant solution obeyed Beer's law with an
extinction coefficient of 0.68104 [20].
2.2 Polymerization
All the solutions, except the DTA solution, were taken in the polymerization tube,
deaerated sufficiently by sparging with nitrogen and were equilibrated at required
temperature. Then the DTA solution, previously temperature每equilibrated, was added instantaneously under nitrogen and the
reaction was allowed to continue for required time intervals. The polymerization was
terminated by adding excess of dilute nitric acid solution so that all the unreacted DTA
was decomposed. The polyacrylamide (PAAM) was precipitated by pouring into excess of
methanol, and then filtered through weighed sintered glass crucible, washed repeatedly
with methanol and dried at 60
The molecular weight of the polymer was determined by viscosity measurement [21] in aqueous solution at 25ºC using an Ubbelohde viscometer and the following Mark-Houwink equation [21]:
[h]=6.80℅10-4
The
2.3 Measurements
The FTIR spectra were taken by the KBr pelletization method using a FTS-40 IR spectrometer (BIO-RAO company, USA). The 1H NMR spectrum was recorded in a JNH-FX100 (JBOX) NMR spectrometer (USA) using CDCl3 as the solvent and TMS as an internal standard.
3. RESULTS AND DISCUSSION
3.1 Rate of polymerization (Rp)
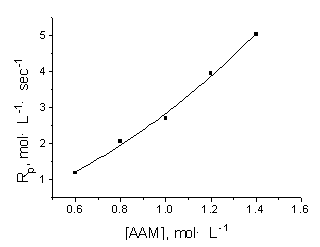 |
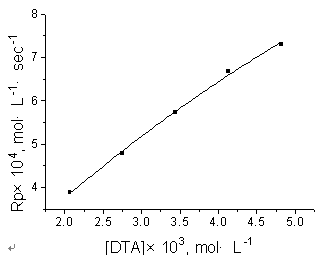 |
| Fig.1 Effect of
[AAM] on Rp [DTA]=1.012℅10-3 mol·L-1, T=273K, pH=13.24 |
Fig.2 Effect of
[DTA] on Rp [AAM]=1mol·L-1, T=273K, pH=13.24 |
The effects of [AAM] and [DTA] on Rp
were studied by varying their concentrations, keeping all the other kinetic factors
constant. It was found that Rp increased with the increase of [AAM] and of
[DTA], as shown in Fig.1 and Fig.2. This is consistent with the general principle of
conventional radical polymerization, i.e. with the increasing concentration of reactants,
the chances of encounters among them increases and the Rp accelerates.
As indicated from their log每log
plots (Fig.3), the rate is found to be proportional to 1.68 and 0.76 orders of [AAM] and
of [DTA], respectively. Therefore, the following relationship is obtained for the initial
concentration of AAM and DTA:
Rp= k [AAM]1.68 [DTA]0.76
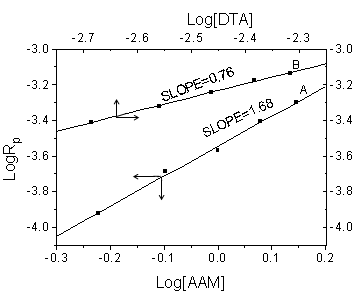 |
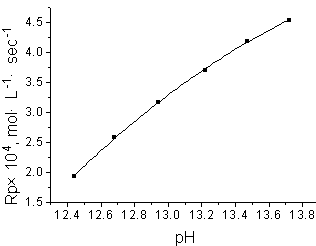 |
| Fig.3 Plots of logRp against log [AAM] (A) and log [DTA] (B)Experimental conditions the same as in Fig.1 and Fig.2, respectively | Fig.4 Effect of pH
on Rp [AAM]=1 mol·L-1,[DTA]=2.065℅10-3 mol·L-1, T=273K |
This means, from the polymerization-rate equation, that
the termination of the growing polymer chains would have both monoradical and biradical
termination mechanisms. On the other hand, the AAM has been employed not only as monomer
taking part in the chain propagation reaction but also as reductant involved in the
initiation.
The effect of pH on Rp was also
studied and the pH value of the reactant solution was regulated with KOH solution. As
shown in Fig.4, with the increase of pH, the Rp exhibited an increasing trend
in the pH range studied. This may be due to the different species and the varying
oxidizability of DTA complex under different pH value. In a lower pH, DTA has a higher
oxidizability and lower stability. Thus, before the radicals initiating the polymerization
of monomer, they may be further oxidized and lost their activity. So, with the decline of
pH, the initiation efficiency of DTA decreases and the Rp becomes lower.
3.2 Effect of temperature
The effect of temperature on the Rp was investigated in the temperature
range 0-25ºC. The Rp increased with the increasing temperature (Fig.5).
From the Arrhenius plot of log Rp against 1/T (Fig.6), the overall activation
energy was calculated as 33.6 kJ/mol.
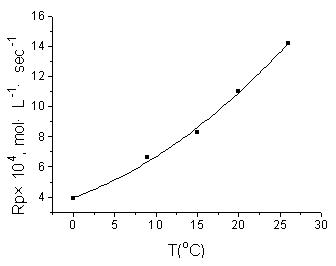 |
Fig.5 Effect of
temperature on Rp [AAM]=1 mol/L, [DTA]=2.065℅10-3 mol·L-1, pH=13.24 |
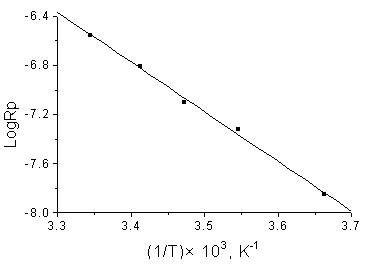 |
Fig.6 Arrhenius plot Experimental conditions the same as in Fig.5 |
3.3 Discussion of the initiation mechanism
To test the nature of the polymerization, free radical inhibitor, atmospheric oxygen
was introduced to the reaction system and the polymerization was inhibited greatly. It
indicated the free radical nature of the reaction.
The proof of initiation mechanism was also obtained from the FTIR
spectrum and 1H NMR spectrum analysis. Due to the difficulty to distinguish the
peaks of 每NH每 and
of 每NH2 in the structure of DTA每initiated PAAM, a poly (methyl methacrylate) (PMMA) sample
initiated by the DTA每formamide redox system has been
obtained to record spectra in order to illustrate the reaction mechanism clearly.
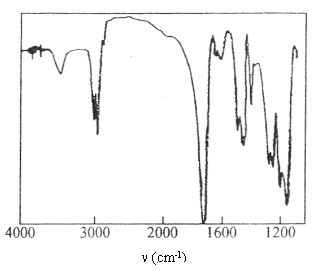
Fig.7 FTIR spectrum of PMMA
The FTIR spectrum of PMMA was shown in Fig.7. It shows the
characteristic absorption bands of imide group i.e. C=Ostr at 1631 cm-1 and
the combined absorption of N- Hdef and C- Nstr at 1600 cm-1.
The single peak at 3450 cm-1 is attributed to N- Hstr. However, all
the above-mentioned peaks do not exist in the IR spectrum (not shown) of
azobisisobutyronitrile (AIBN)每initiated PMMA.
At the same time, the proton signal of 每NH每
also appeared at 5.7 ppm in the 1H NMR spectrum of
the DTA每formamide initiated PMMA sample
(Fig.8).
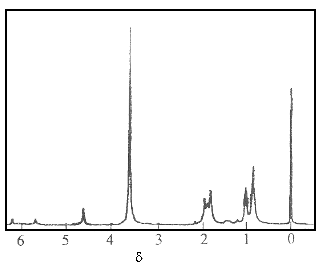
Fig.8 1H NMR spectrum of PMMA in CDCl3
Based on the above
discussions, it can be concluded that the DTA每PAAM
(每NH2) redox couple as initiator
has initiated the radical polymerization. The mechanism is proposed as follows: one
electron was transferred to Ag (III) from the nitrogen of amide group and forms a radical
cation, which tends to lose the proton in alkaline medium and turn into a free radical to
initiate the polymerization. This was in agreement with the conclusion [13] derived
from the AAM polymerization initiated by DPA每AAM
redox initiator.
In addition, in the oxidations by the DTA [Ag (III)], Ag (II), which is
generated in the slow step and is instable in an alkaline medium, disproportionates
quickly to give Ag (III) and Ag (I) [4,5]. So, a tentative initiation mechanism
which involved in a two-step single-electron-transfer process of DTA is shown as follows:
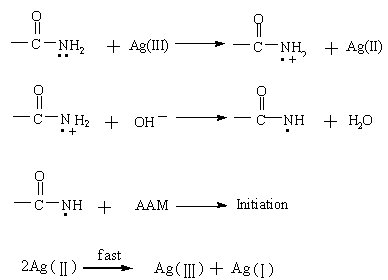
4. CONCLUSION
Generally, it is believed that the oxidation by DTA is a two-electron-transfer
process, without radicals produced and DTA can not initiate polymerization of vinyl
monomer. However, under our experimental conditions, DTA employed as oxidant and AAM
itself as reductant formed a redox initiation system and initiated the homopolymerization
of AAM successfully in aqueous alkaline medium. Thus a two-step
single-electron-transfer mechanism is proposed during the reduction of DTA. This is
similar to the mechanism established in the radical polymerization initiated by DPA
(III)-reductant initiator in our previous studies [13-18].
Moreover, it is found that DTA-AAM is a promising initiator. It exhibited higher Rp than other
redox initiators. The polymerization reaction can be carried out at a mild temperature due
to the lower activation energy and in an aqueous alkaline medium. The proof of initiation
mechanism was obtained from FTIR and 1H NMR tests. In summary, the DTA-AAM is concluded to be an effective redox initiator and exhibits an
excellent performance in radical polymerization.
REFERENCES
[1] Jaiswal P K, Yadava K L. Talanta, 1970, 17 (3): 236.
[2] Jaiswal P K. Microchem. J., 1970, 15 (2): 205.
[3] Jaiswal P K. Analysis, 1972, 1 (7): 503.
[4] Sen Gupta K K, Nandy B K, Sen Gupta S. J. Org. Chem., 1994, 59 (4): 858.
[5] Sen Gupta K K, Nandy B K, Sen Gupta S. J. Chem. Soc., Perkin Trans. 2, 1993 (4): 767.
[6] Raviprasad T, Sethuram B, Navaneeth Rao T. Indian J. Chem., Sect. A, 1979, 18A (1):
40.
[7] Sen Gupta K K, Nandy B K, Sen Gupta S. Indian J. Chem., Sect. A, 1997, 36A (3): 190.
[8] Raviprasad T, Sethuran B, Navaneeth Rao T. Indian J. Chem., Sect. A, 1980, 19A (3):
261.
[9] Raviprasad T, Sethuran B, Navaneeth Rao T. Oxid. Commun., 1988, 11 (3-4): 133.
[10] Raviprasad T, Sethuran B, Navaneeth Rao T. Indian J. Chem., Sect. A, 1982, 21A (2):
169.
[11] Sen Gupta K K, Nandy B K, Sen Gupta S. J. Chem. Res., Synop, 1993, (10): 396.
[12] Shan J H, Wang L, Shen S G et al. Chem. Res. Chin. Univ., 2000, 16 (3): 218.
[13] Liu Y H, Song M F, Hou R S. Chem. J. Chin. Univ., 1992, 13 (8): 1151.
[14] Song X R, Liu Y H, Feng X F et al. J. Hebei Univ., 1996, 16 (1): 32.
[15] Liu Y H, Zhang J S, Li W P et al. Polymer Materials Science and Engineering, 2002, 18
(1): 161.
[16] Song X R, Liu Y H, Liu W H et al. Polymer Materials Science and Engineering, 1999, 15
(2): 162.
[17] Liu Y H, Shang Y J, Yu T L et al. J. Hebei Univ., 1997, 17 (3): 30.
[18] Liu Y H, Liu W H, Zhao M et al. Acta Polym Sinica, 1997, 5: 597.
[19] Balikungeri A, Pelletier M, Monnier D. Inorg. Chim. Acta, 1977,22: 7.
[20] Jensovsky L. Collect Czech Chem. Commun., 1967, 32 (5): 1996.
[21] Ishige T, Hamielec A E. J Appl Polym Sci, 1973, 17: 1479.