http://www.chemistrymag.org/cji/2004/062013pe.htm |
Feb.21, 2004 Vol.6 No.2 P.13 Copyright |
Curing and thermal property
of boron-containing o-cresol formaldehyde resin
Xia Liya, Gao Jungang, Yu Zhenxia
(Supervision Institution of Quality&Technology, College of Chemistry and Environmental
Science, Hebei University, Baoding 071002, China)
Received Dec. 7, 2003.
Abstract The curing and thermal degradation process of boron-containing
o-cresol formaldehyde resin (BOCNR) was studied by infrared spectroscopy (IR) and
thermogravimetry analysis (TGA). The results show that hexatomic ring containing
coordinate linkage of boron-oxygen formed in the curing process of BOCNR, and the
coordinated oxygen atom was offered by phenol hydroxyl. TGA results show that BOCNR have
good thermostability and its degradation processes can be divided into three stages. In
the second and third stages, the decomposition reactions are all following first mechanism
function.
Keyword Boron-containing phenol-formaldehyde resin, o-cresol, Boric acid,
Thermal analysis
1. INTRODUCTION
The boron-containing phenol-formaldehyde resin is a modified phenolic resin, which is
obtained by introducing boron to the main chain of common phenol-formaldehyde resin. This
resin can be converted into a three-dimentional cross-linked thermoset network by
self-cross-linking reaction during curing process, so it has many excellent performances,
such as thermostability, mechanical strength, electric properties and defence of neutron
radiation. It is suitable for manufacturing laminated and moldable composite materials,
insulated materials, ablation and abrasion resistant materials. With the variation of the
raw materials used in the synthesis process, various type of boron-containing
phenol-formaldehyde resins have been reported, such as boron-containing
phenol-formaldehyde resin (BPFR), boron-containing bisphenol-A formaldehyde resin (BBPFAR)
[1-4]. While the synthetic structure and thermal properties of boron-containing
o-cresol formaldehyde resin (BOCNR) have not been investigated.
In this work, the structure changes of BOCNR during curing were
monitored by Fourier-transform infrared (FTIR) spectrometry; the weight changes and
degradation kinetics were studied by thermogravimetry analysis (TGA).
2. EXPERIMENTAL
2.1 Materials
O-cresol (OCN), Boric acid, 37% aqueous formalin, acetone and sodium hydroxide were all
analytically pure grade, which were supplied by Tianjin Chemical Reagent Co. of China.
2.2 Synthesis of BOCNR
O-cresol, aqueous formalin and NaOH were introduced into a three-necked flask, equipped
with a stirrer, a thermometer and a condenser. The mixture was stirred and heated to 70oC,
then the reaction was maintained at this temperature for 1h. When the water was removed in
vacuum, salicylalcohol of o-cresol was obtained. In the second step, boric acid was added
to this system, heated to 102-110oC and
held the temperature in the above range for 45min .Then the water formed in the reaction
was removed in vacuum. Finally the yellow solid BOCNR was obtained.
2.3 Infrared spectrum analysis
A Fourier-transform infrared (FTIR) spectrometer (Bro-Rad FTS-40 USA) was used to
investigate the structure changes of the BOCNR during the curing and thermal degradation.
The BOCNR was dissolved in acetone and then coated as a thin film on a potassium bromide
plate. When the solvent in the film had completely evaporated in vacuum, the potassium
bromide plate was scanned by the FTIR instrument. Then it was scanned after being cured at
different temperatures. The principal absorption bands appear as follows [4,5]:
the benzene ring is at 1600cm-1, the borate B-O is at 1350cm-1,
phenol hydroxyl C-O is at 1250cm-1, the -CH2- group appears at
1450cm-1, methylol group is at 1020cm-1, ether linkage C-O is at
1100cm-1, carbonyl group is at 1650cm-1. Quantitative analysis was
doing according to the literature [5]. The benzene ring absorption at 1600cm-1
was used as internal standard. According to the Beer-lambert law A=lgI/I0, the ratios of
absorbance A1350/A 1600(borate value), A1250/A1600 (phenol hydroxyl value), A1100/A1600 (ether value),
A1020/A1600 (methylol value), A1650/A1600 (carbonyl value) were obtained.
2.4 Thermal analysis
A Shimadzu TGA-40 JP thermogravimetric apparatus was used to determine the weight loss
behaviour of BOCNR during degradation. About 8mg BOCNR powder cured at 180℃ for 4h was introduced into the thermo-balance, then heated to 900oC
at 10oC/min heating rate in air.
3. RESULT AND DISCUSSION
3.1. Structure of boron-containing o-cresol formaldehyde resin
The process of synthesizing BOCNR by the method of formalin was divided into two steps.
Salicylalcohol of OCN was formed in the first step, methylol groups were mainly at ortho
and para positions of the phenyl ring [6], and then it reacted with boric acid. According
to the literature [3], the reactivity of methylol group with boric acid was higher than
that of phenol hydroxyl. So in the second step, the reaction of boric acid with methylol
group is prior to that of boric acid with phenol hydroxyl. The reaction can be described
as Scheme 1.

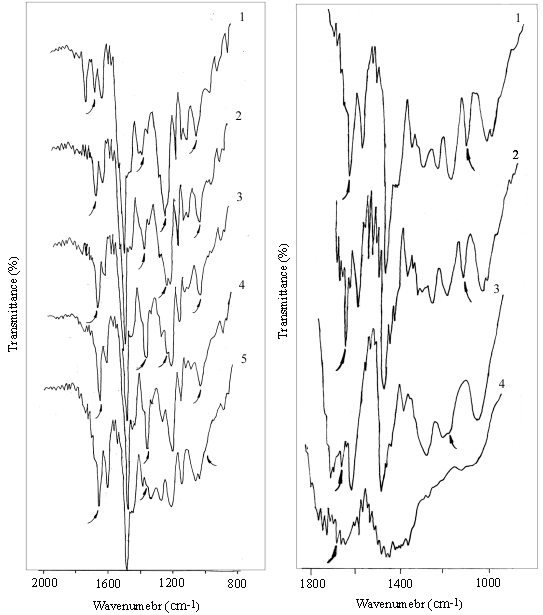
Fig.1 and Table 1 show the IR absorption
variation of BOCNR during the curing reaction. As it can be seen from Fig.1 and Table 1,
below 160oC, the absorption of borate B-O linkage was increased with the rising
of curing temperature, while, the absorption of methylol groups and phenol hydoxyls were
decreased. This is caused by the reaction between methylol group and phenol hydroxyl group
with unreacted -OH groups in boric acid. Since most of methylol groups had been reacted in
the synthesizing process, the reaction of phenol hydroxyl group with unreacted -OH groups
inboric acid is the main reaction. This can also be proved by the disappearance of phenol
hydroxyl group at 160oC. So the main reaction in the curing process is
described as Scheme 2.

Table1 Changes of functional group values of
BOCNR during curing process
Curing condition T(oC) |
Carbonyl value |
Borate value |
Phenol hydroxyl value |
Methylol value |
uncured |
0.06 |
0.50 |
0.62 |
0.16 |
120 |
0.10 |
0.64 |
0.48 |
0.14 |
130 |
0.10 |
0.77 |
0.46 |
0.13 |
140 |
0.16 |
0.81 |
0.45 |
0.13 |
160 |
0.18 |
0.92 |
0.0 |
- |
200 |
0.29 |
0.64 |
0.0 |
- |
220 |
0.49 |
0.65 |
0.0 |
- |
According to the
literature [1,3 ], in the curing resin, when the hexatomic ring containing B←O coordination bond formed, the IR absorption band of B-O borate at
1350cm-1 would disappear. As shown in Fig.1 and Table 1, the borate value decreased after
cured at 160oC. This showed that the hexatomic ring structure containing B←O coordination bond formed at higher temperatures and the
coordinated oxygen was offered by phenol hydroxyl because most of methylol groups had been
reacted. The reaction and final molecular structure may be described as Scheme 3.

|
||
Fig.1 Infrared spectrum of BOCNR in curing process (1) uncured, (2) 120oC, (3) 140oC, (5) 160oC, (6) 200oC cured 0.5h |
Fig.2 Infrared spectrum of BOCNR in thermal degradation process (1) 200oC, (2) 220oC, (3) 300oC, (4) 400oC decomposed 0.5h |
|
3.2 Thermal stability and degradation
kinetics of BOCNR
As shown in Fig.1, the spectra had an ether linkage absorption peak at 1050cm-1,
formed from condensation reaction of benzyl hydroxyl groups. The ether linkage was
oxidized at higher temperature to form a carbonyl group. The carbonyl value of uncured
resin is only 0.06, however, after it was cured at 160oC, the carbonyl value is
increased to 0.18. The reaction is described as Scheme 4.

Fig.2 shows the IR absorption variation of BOCNR during thermal
degradation. As it can be seen from Fig.2, with the rising temperature, the absorption
peak of ether linkage at 1100cm-1 decreases first, then the absorption of carbonyl group
decreases gradually. At 300oC about 1h, the
absorption of carbonyl group disappears, while the absorption of -CH2- (at
1430cm-1) and the benzene ring are very strong. So ether linkage and carbonyl
group in the resin intensely affect the thermal stability of BOCNR.
A Shimadzu TGA-40 thermogravimrter was used to determine the weight
loss behaviour of BOCNR. As shown from Fig.3, the common phenol-formaldehyde resin (PFR)
has higher weight loss rates than that of BOCNR. The weight loss for common PFR is over
99% at 580oC, while the BOCNR is only 34.7 % at 580oC. The
temperature of semi-weight loss is about 227oC higher than that of common PFR,
and the start temperature of weight loss (280oC) is about 50oC
higher than that of PFR. The thermostability of BOCNR is
close to that of boron-containing bisphenal-A formaldehyde resin (BBPFAR)[3]
and BPFR[7]. The start temperatures of weight loss of BBPFAR and BPFR are 310oC
and 315oC respectively.
And the semi-weight loss of BBPFAR and BPFR are all at 580oC which are lower than that of BOCNR.
According to the TGA curves (Fig.3), the degradation process can be
divided into three stages. In the first stage (about 260-417oC), the total
weight loss for BOCNR resin at the 10oC/min heating rate is about 4.56% , which
is caused by the evaporation of water and small molecules. In the second stage (417-580oC)
and third stage (580-900oC), the weight losses are 30% and 32% respectively.
Related with structure changes shown in Fig.2, the weight loss in the second stage may be
caused by the oxidation and breakage of ether linkages and carbonyl groups. In the third
stage, -CH2- group、borate B-O linkage、benzene ring may be oxidized and broken.
The following kinetic equation was assumed to hold for the reaction [8,9]
![]()
where A is the pre-exponential factor in the Arrhenius
equation, E is the apparent activation energy, R is the universal gas constant, j is the heating rate, T is absolute
temperature, and G(a) is the integral form of the conversion dependence function. The
correct form of G(a) depends on the proper mechanism of the decomposition reaction [9].
Different expressions of G(a) for some solid-state reaction mechanisms can be
described as follows: first order, G(a) is -ln(1-a); second order, G(a) is 1/(1-a); third
order, G(a) is 1/(1-a)2 .
According to the above equation, the activation energy can be obtained
at different heating rates from fitting the ln[G(a)/T] versus 1/T plots. For different
degradation stages, the apparent activation energies and pre-exponential factors were all
tested for different mechanism functions. The results are listed in Table 2.
Table 2 Kinetic parameters of thermal degradation of BOCNR for different
mechanism function at 10oC/min heating rate
Reaction order |
Correlation coefficient (r) |
DE (kJ/mol) |
lnA (s-1) |
Standard deviation |
|
Second stage |
1 |
0.9911 |
276.3 |
52.8 |
0.0614 |
2 |
0.9672 |
420.1 |
62.7 |
0.3196 |
|
3 |
0.8981 |
520.4 |
79.8 |
0.7432 |
|
Third stage |
1 |
0.9931 |
123.2 |
25.8 |
0.0578 |
2 |
0.9426 |
137.3 |
14.2 |
0.2859 |
|
3 |
0.785 |
131.6 |
15.3 |
0.6447 |
As shown in the Table 2,
for the same degradation stage at a given heating rate, the correlation values for
different mechanisms are different. According to the principle that the probable mechanism
has high correlation coefficient value and low standard deviation value, the mechanism
function and other kinetic parameters can be obtained, and the results are listed in Table
3.
Table 3 Kinetic parameters of thermal degradation of BOCNR at 10oC/min
heating rate
Reaction order |
Correlation coefficient (r) |
DE (kJ/mol) | lnA (s-1) |
Standard deviation |
|
Second stage |
1 |
0.9911 |
276.3 |
52.8 |
0.0614 |
Third stage |
1 |
0.9931 |
123.2 |
25.8 |
0.0578 |
4. CONCLUSIONS
During the curing process of BOCNR, borate B-O group and hexatomic ring containing
coordinate linkage of boron-oxygen was formed and the coordinated oxygen atom was offered
by phenol hydroxyl. Thermal degradation of BOCNR begins with the oxidation and breakage of
ether linkage and carbonyl group. The concentration of phenol hydroxyl, methylol group and
carbonyl group in the cured resin is the most important factor that affected the
thermostabilitis of BOCNR. The thermostabilitis of BOCNR are more excellent than that of
common PFR.
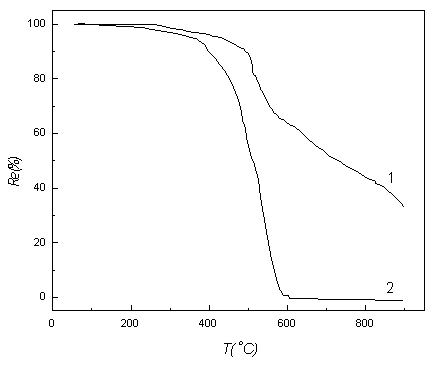
Fig.3 Thermogravimetric analysis (1) BOCNR, (2) PFR, at heating rate of
10oC/min in air
The TGA results show that the decomposition process of BOCNR can be
divided into three stages, and in the second and third stage the decomposition reactions
all follows first reaction order.
REFERENCES
[1] America Patash Chem. Corp. British Patent, 957611,1964.
[2] Heelfel H B, Kiessling H Y, Lamper F. Schoenrogge B. Ger. offen, 2,436,359,
1975.
[3] Gao J G, Liu Y F, Wang F L. Eur. Polym. J, 2001, 37: 207-210.
[4] Gao J G, Liu Y F. J. Applied Polymer Science, 2000, 76: 1054-1061.
[5] Shen D Y. Application of infrared spectrum in polymer. Beijing: Science Press, 1982,
91.
[6] Hu H W. Organic Chemistry. Beijing: Higher Education Press, 1990, 203-205.
[7] Gao J G, Liu Y F, Yang L T. Polymer Degradation and Stability, 1998, 0, 1-4.
[8] Liu Z H. Indrodution of thermal analysis. Beijing: Chemical Industry Publishing Co.
1991, 100-110.
[9] Madhusudanan P M, Krishnan K, Ninan K N. Thermochim.Acta., 1986, 97: 189-201.