http://www.chemistrymag.org/cji/2004/065032ne.htm |
May 2,
2004 Vol.6 No.5 P.32 Copyright |
Zhu Liya 1,2, Hu Qiufen 2,
Yang Guangyu 2, Yin Jiayuan 2
(
Received Jan. 15, 2004; Supported by Invention Foundation of Kunming Institute of Precious Metals (NO: 2002-BY-01) and Natural Science Foundation of Yunnan Province (0111143)
Abstract A new chromogenic reagent, 2-(2-quinolylazo)-5-dimethylaminoaniline (QADMAA) was synthesized. A sensitive, selective and rapid method for the determination of palladium based on the rapid reaction of palladium(II) with QADMAA was developed. In the presence of 0.5-2.5 mol/L of hydrochloric acid solution and cetyl trimethylammonium bromide (CTMAB) medium, QADMAA reacts with palladium to form a violet complex of a molar ratio 1:2 (palladium to QADMAA). The molar absorptivity of the chelate is 1.35¡Á105 L.mol-1.cm-1 at 600 nm. Beer's law is obeyed in the range of 0.01- 0.6 m g/ml. The relative standard deviation for eleven replicate sample of 0.2 m g/ml level is 1.02 %. This method had been applied to the determination of palladium with good results.Keywords 2-(2-quinolylazo)-5-dimethylaminoaniline; palladium; spectrophotometry
Palladium is an important element for use in
industry and biological systems as well [1-2]. Many sensitive instruments, such
as spectrofluorimetry, X-ray fluorescence spectrometry, neutron activation analysis,
atomic absorption spectrometry, chemiluminescence, and the like have widely been applied
to the determination of palladium. But the spectrophotometric method still has the
advantages of being simple and not requiring expensive or complicated test equipment. For
this reason, a wide variety of spectrophotometric methods for the determination of
palladium have been developed [3-6].
In our previous work, some 2-quinolylazo-phenol reagents were reported
for the determination of metal ions [7-9]. This kind reagent has a higher
sensitivity than pyridylazo reagents because of its larger conjugated system. However, the
2-quinolylazo-phenol reagent has also a disadvantage of its poor selectivity because both
the oxygen atoms and nitrogen atoms donate to the metal ions. To select a more sensitive
and selective reagent, we synthesized 2-(2-quinolinylazo)-5-dimethylaminoaniline (QADMAA)
and studied the color reaction of QADMAA with palladium in detail. This reagent has a
higher selectivity than 2-quinolylazo-phenol reagents because it only donates nitrogen
atoms to metal ions. Based on the color reaction of QADMAA with palladium, a highly
sensitive, selective and rapid method for the determination of palladium was
developed.
1.1 Apparatus
A UV-160 A spectrophotometer (Shimidzu Corporation, Tokyo, Japan) equipped with 1 cm cells was used for all absorbance measurements. The pH values were determined with a Beckman F-200 pH meter (Beckman Instruments, Fullerton, CA, USA).
1.2 Synthesis of QADMAA
2-Aminoquinoline (6.9 g) was dissolved in 500 mL anhydrous ethanol. To which, sodamide (2.0 g) was added and the mixture was refluxed in boiling water bath for 5 h, followed by the addition of isoamyl nitrite (7.4 mL). The solution was refluxed for 30 min with boiling water bath. The solution was cooled and placed over night at 0ºC. The diazo salt was obtained by filtering this solution with an isolation yield of 95%. The diazo salt was dissolved in 200 mL anhydrous ethanol, followed by the addition of m-dimethylaminoaniline (5.7g; 0.042 mol). The carbon dioxide was ventilated into the solution with stirring until the pH reaches to 8.0. The solution stood for two days, and evaporated to dryness. The residue was re-crystallized with 30% ethanol. QADMAA was obtained with 36% yield. The structure of QADMAA was verified by elemental analysis, IR, 1HNMR and MS. Elemental analysis: C17H17N5 found (calculated) C 69.82 (70.08), N 23.83 (24.04), H 6.04 (5.88). IR (KBr) (cm-1): 3300 (n N-H), 1600, 1560, 1505, 1426
(nC=C,N=N), 1380, 1323 (n C-N), 2850 (n C-H), 1465 (n C-H), 3050, 3015 (n Ar-H), 1175, 1120, 865, 780, 720 (n Ar-H). 1HNMR (solvent: d6-acetone) (d ppm): 2.28 (s, 6H, N-CH3), 3.69 (s, 2H, -N-H2); 6.86- 7.85 (m, 9H, Ar-H). MS: 291 (M+). All these show that the QADMAA has the structure in Fig.1.
1.3 Reagents
All of the solutions were prepared with ultra-pure water obtained from a Milli-Q50 SP Reagent Water System (Millipore Corporation, USA). A 5¡Á10-4 mol/L of QADMAA solution was prepared by dissolving QADMAA with 95% of ethanol. A stock standard solution of palladium (1.0 mg/mL) was obtained from Chinese Standard Center, and a work solution of 2.0 m g/mL was prepared by diluting this solution. A 5 mol/L of hydrochloric acid was used. Cetyl trimethylammonium bromide (CTMAB) solution (1.0 % (w/v)) was prepared by dissolving CTMAB with 20% ethanol. All chemicals used were of analytical grade unless otherwise stated.
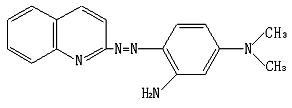
Fig.1 The structure of QADMAA
1.4 General procedure
To a standard or sample solution containing no more than 15 m g of Pd(II) in a 25 mL
of calibrated flask, 5 mL of 5 mol/L hydrochloric acid, 4.0 mL of 5¡Á10-4 mol/L
QADMAA solution and 2.0 mL of 1.0 % CTMAB solution were added. The mixture was diluted to
volume of 25 mL and mixed well. After 10 min, the absorbance was measured in a 1 cm cell
at 600 nm against a reagent blank prepared in a similar way without palladium.
1.5 Application
The proposed method has been successfully applied to the determination of palladium in
water and catalyst.
For water sample, taking an appropriate volume (planting effluents 20
ml, river water 500 ml) of water sample in a 500 mL flask. The samples were concentrated
to about 10 mL by heating on a hot plate. 5 mL concentrated nitric acid and 2 mL of 30%
hydrogen peroxide were added in this solution. The mixture was heated on a hot plate and
evaporated to near dryness. The residue was dissolved with 5 mL of 5% hydrochloric acid
and transferred into a calibrated flask, and 2 mL of 10% citric acid was added to mask the
nickel and cobalt. The solution was neutralized with sodium hydroxide, and analyzed by
general procedure. The recovery of palladium was determined by adding 1.0 and 4.0 m g of
palladium to water samples. A standard method using atomic absorption spectrometry was
also used as a reference method. The results are shown in Table1.
Table 1 Determination of palladium in the water sample
Samples |
AAS method |
Found |
RSD% (n=5) |
Pd(II) added (m g) |
Pd(II) Found (m g) |
Recovery (%) (n=5) |
River water |
0.0182 (m g/mL) |
0.0156 (m g/mL) |
2.3 |
1.0 (4.0) |
1.03 (4.03) |
103 (101) |
Planting effluents |
0.248 (m g/mL) |
0.237 (m g/mL) |
2.1 |
1.0 (4.0) |
0.96 (4.13) |
96 (103) |
| catalyst | 0.532 (%) |
0.536 (%) |
1.2 |
1.0 (4.0) |
1.01 (3.94) |
101 (98) |
For catalyst, 0.1 g of samples was weighed accurately into the Teflon high-pressure microwave acid-digestion bomb (Fei Yue, Analytical Instrument Factory, Shanghai, China). 3.0 ml of concentrated nitric acid, 2 mL of hydrochloric acid and 5.0 ml of 30% hydrogen peroxide were added. The bombs were sealed tightly and then positioned in the carousel of the microwave oven (Model WL 5001, 1000 W, Fei Yue Analytical Instrument Factory, Shanghai, China). The system was operated at full power for 10 min. The digest was evaporated to near dryness. The residue was dissolved with 10 ml of 10% of hydrochloric acid, then transferred into a 50 ml of calibrated flask and diluted to volume with 10% of hydrochloric acid. The palladium content was analyzed by general procedure.
2 RESULTS AND DISCUSSION
2.1 Absorption Spectra
The absorption spectra of QADMAA and its Pd(II) complex are shown in Fig.2. The absorption
peaks of QADMAA and its complex are located at 440 nm and 600 nm.
2.2 Effect of Acidity
Results showed that the optimal condition for the reaction of Pd(II) with QADMAA is in the
acid medium. Therefore, the effect of hydrochloric acid, sulfuric acid, perchloric acid,
phosphoric acid and the like, on the color reaction of Pd(II) with QADMAA was studied.
Experiment shows that hydrochloric acid has the best effect, and the concentration of
hydrochloric acid within 0.5-2.5 mol/L was found to give a maximum and constant
absorbance. So 5 ml of 5 mol/L of hydrochloric acid was recommended.
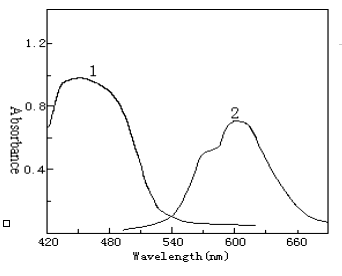
Fig.2 Absorption spectra of QADMAA
and its Pd(II) complex
1 QADMAA-CTMAB blank against water; 2 QADMAA-Pd(II)-CTMAB complex against reagent blank
Conditions: Pd(II) 4.55´ 10-6 mol/L, QADMAA 3.64´ 10-4
mol/L
2.3 Effect of surfactants
The effect of surfactants on Pd(II)-QADMAA chromogenic system was studied. In the presence
of cationic surfactants or nonionic surfactants medium, the absorption of the chromogenic
system increases markedly. Experiments show that CTMAB is the best additive. The use of
1.0 % CTMAB solution 1- 4 mL give a constant and maximum absorbance. Accordingly, the use
of 2 mL was recommended.
2.4 Effect of QADMAA concentration
For up to 15 m g of Pd(II), the use of about 3.5-5 mL of 5¡Á10-4 mol/L of
QADMAA solution has been found to be sufficient for a complete reaction. Accordingly, 4 ml
of QADMAA solution was added in all further measurement.
2.5 Stability of the chromogenic system
After mixing the components, the absorbance reaches its maximum within 8 min at room
temperature and remains stable for 6 h in aqueous solution. The chelates are stable for at
least 20 h.
2.6 Calibration Curve and Sensitivity
The calibration curve show that Beer's law is obeyed in the concentration range of 0.01-
0.6 m g Pd(II) per mL, The linear regression equation obtained was: A = 1.294 C (m
g/mL) + 0.0158, (r=0.9996). The molar absorptivity was calculated to be 1.35¡Á105
l.mol-1.cm-1 at 600 nm. The relative standard deviation at a
concentration level of 0.2 m g Pd(II) per mL (11 repeat determinations) was 1.02 %.
2.7 Composition of the complex
The composition of the complex was determined by molar ratio method(Fig.3) and continuous
variation method (Fig.4). Both showed that the molar ratio of Pd(II) to QADMAA is 1:2.
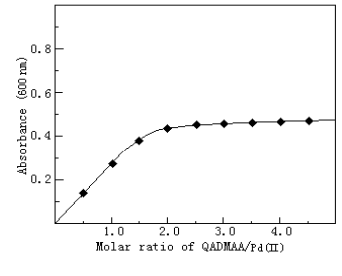
Fig.3 Composition of Pd(1I)-QADMAA complex by molar ratio method
The concentration Pd(II) was 0.3¡Á105 mol.L-1, other conditions as
in standard procedure
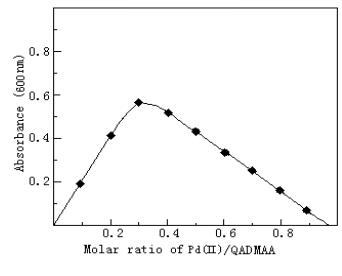
Fig.4 Composition of Pd(I1)-QADMAA complex by continuous variation method
The concentration of Pd(II)+QADMAA was 1.0¡Á105
mol.L-1, other conditions as in standard procedure
2.8 Interference
The selectivity of the proposed method was investigated by the determination 5 m g/25ml of
Pd(II) in the presence of various ions within a relative error of ¡À 5% are given in Table
2.
Table 2 Tolerance limits for the determination of 5 m g of Pd(II) with QADMAA (relative error ¡À 5% )
Ion added |
Tolerate (mg) |
NO3-, K+, borate, Na, Cl-, Mg2+, SO42-, ClO4- |
200 |
Li+, Al3+, PO43-, NO2-, ClO3- |
20 |
Ca2+, Sr2+, IO3-, BrO3-, B(III) |
10 |
Mn2+, Ce(IV) , Fe3+, Mo(VI), V(V) |
5 |
Ti(IV), Bi(III), Cr(VI), Ba2+ W(WI), U(IV), [Co2+]* |
1 |
| Cd2+, Pd2+, Cr3+, La3+, Zn2+, Zr(IV), [Ni2+]* | 0.5 |
| Bi(III), Pb2+, Hg 2+, Sb3+, Th(IV), Sn(IV) | 0.1 |
Se(IV), Te(IV), Au3+, Cu2+, Ag+ |
0.05 |
Ni2+, Co2+ |
0.01 |
| * masked with 2 mL of 10% citric acid | |
REFERENCE
[2] Patrick G, Michel P. Platinum Metals Review. 2003, 47 (2): 60-72.
[3] Maria B, Katarzyna P. Chemia Analityczna. 2003, 48 (1): 87-95.
[4] Sarma L S, Kumar J R, Reddy K J, Reddy A V. Analytical Sciences. 2002, 18 (11): 1257-1261.
[5] Ma D L, Cui F L, Xia D S, Wang Y L. Analytical Letters. 2002, 35 (2): 413-421.
[6] Gholivand D, Mohammad B. Talanta. 2000, 52 (6): 1055-1060.
[7] Hu Q F, Yang G Y, Yin J Y. Journal of Environment Monitoring. 2002, 4 (6), 956-959.
[8] Yang G Y, Hu Q F, Yang G Y, Huang Z J, Yin J Y. Analytical Sciences. 2003, 19 (2): 299-302.
[9] Yang G Y, Huang Z J, Hu Q F, Yin J Y. Talanta. 2002, 58 (3): 511-515. ¡¡