http://www.chemistrymag.org/cji/2004/065037pe.htm |
May 2,
2004 Vol.6 No.5 P.37 Copyright |
Qian Chen, Diao Guowang
(College of chemistry and chemical engineering,
Yangzhou University, Yangzhou 225002, China)
Received Mar. 2, 2004; Supported by the National Natural Science Foundation of China (Grant No. 20373060)
Abstract The inclusion complex of b-cyclodextrin(b-CD) with 4-(2-pyridylazo)resorcinol(PAR) has been synthesized and characterized by UV-vis spectroscopy, FT-IR, and powder X-ray diffractometry. The results indicate that the ratio of host to guest in the inclusion complex is 2:1, which means that PAR molecule is enwrapped from both ends by the hydrophobic cavities of two b-CDs. At 25ºC, the dissociation constant, KD, of the inclusion complex is 3.5¡Á10-6mol·dm-3.Keywords Superamolecule, 4-(2-pyridylazo)resorcinol, Inclusion complex, b-Cyclodextrin 1. INTRODUCTION
Cyclodextrins(CDs) are cyclic glucopyranose oligomers bearing a characteristic toroidal shape. Normally, CDs are cyclic oligomers of six, seven, or eight head to end linked a-D-glucopyranose units, denoted a-, b-, and g-CDs, respectively. The surface of the molecule is hydrophilic, while the cavity is relatively hydrophobic. Owing to their structural characteristics, CDs are capable of forming inclusion complexes with a variety of guest molecules by incorporating them within their hydrophobic cavities, and the properties of the inclusion complexes have been extensively studied[1-3].
CDs' capability of forming inclusion complexes could be applied to reduce pollution, due to the stable inclusion complexes of CDs and pollutants. Since 1990s', the study of CDs for controlling pollution has been applied in practice step by step. Murai et al[4] reported the reclaiming of the nonionic surfactants in water with b-CD polymers. Sheremata et al[5] studied cyclodextrins for desorption and solubilization of 2,4,6-trinitrotoluene and its metabolites from soil. Brusseau et al[6] used cyclodextrins as a solubility-enhancement agent for remediation of a tetrachloroethene-contaminated aquifer. However, some approaches must be applied to decompose the pollutants, and the effect of cyclodextrins on the decomposing process of the pollutants has aroused great interests of the environmental scientists.
4-(2-pyridylazo)resorcinol(PAR) is widely used as indicator, and azo dyestuff. PAR is insoluable in water, but easily dissolved in organic solvents like alcohol, ether, etc[7]. The PAR molecule, consists a pyridyl group which strongly constrains its biotic decomposition, and a resorcinol group which causes pollution. As to other azo dyestuffs, it is difficult to dispose pollutants in their application. Actually, pollution caused by dyes is one of the major hazards to environment. Nowadays, the pollutants are normally treated by chemical, biochemical and physical methods and the processes are usually complicated. Sometimes, it requires expensive equipments. In this article, the thermodynamics of the inclusion complex of b-CD with PAR with spectroscopy have been investigated. 2. EXPERIMENTAL
2.1 Reagents
b-CD(Shanghai Chemical Reagents Company) was twice recrystallized before using. PAR (A.R., Shanghai Chemical Factory) and other chemical reagents were of analytical pure and used without further purification. Double distilled water was used to prepare all solutions.
2.2 Apparatus
To determine the dissociation constant of the inclusion complex, UV-vis spectra were taken between 200-600nm using a UV-2501(Shimazu) double-beam spectrophotometer with a stoppered quartz cell of 1.00 cm path length. The IR spectra were taken by a FTIR spectrometer (Nicolet 740) with KBr films. Powder X-ray diffractospectra were taken by a M03XFH (MAC Science) diffractometer. 3 RESULTS AND DISCUSSION
As described before, PAR is difficult to be dissolved in water. In our experiments, PAR aqueous solution was prepared with excess addition of solid PAR and vigorously stirring of the mixture. The clear solution was obtained by filtering. The concentration of PAR was determined by UV-vis spectroscopy. The molar absorption coefficient of PAR at 25ºC was determined as follows: 0.01mmol PAR was dissolved in 10mL ethyl alcohol. Then a given amount of the solution was taken with a micro-injector and injected into a given amount of water to form PAR aqueous solution. The UV-vis spectra of the PAR aqueous solution were recorded and shown in Fig.1. At 25ºC, the wavelength of maximum absorption is 408 nm. From the peak intensity, the molar absorption coefficient can be calculated. The average value of the molar absorption coefficient, e , of PAR in aqueous solution is 1.82¡Á104 mol·dm-3·cm-1.

Fig.1 At 25ºC, UV spectra of PAR aqueous solution with different concentrations of PAR.
cPAR/10-6 mol·L-1: a.5.0; b.8.0; c.10; d.15; e.20; f.25; g.30
3.1 Formation of PAR-(b-CD)n inclusion complex and
its dissociation constant
Fig.2 shows the UV-vis spectra of 2¡Á10-5 mol·L-1 PAR with
different amounts of b-CD.
It can be seen that with the addition of b-CD, the UV-vis absorption peak shifts in hypsochromic direction,
which may be due to the transportation of the PAR molecule from polar solvent (water) into
the relatively apolar cavity of cyclodextrin to form inclusion complex, which corresponds
to the p->p*
transition in PAR molecule. Meanwhile, the intensity of UV-vis absorption decreases while
the concentration of b-CD
increasing.

Fig.2 UV spectra of PAR aqueous solution
with different amounts of b-CD
at 25ºC.
cPAR/10-5 mol·L-1£º2.0, cb-CD/10-3 mol·L-1£º
a.0£»b.0.5£»c.1.0£»d.2.0£»e.3.0£»f.4.0£»g.5.0£»h.7.0
In fact, the PAR monomers
and the inclusion complexes coexisted in the solution, therefore, UV-vis absorption shown
in Fig.2 with different b-CD concentration is the superposition of those occurred by PAR
monomers and PAR-(b-CD)n
inclusion complexes, where n is the number of b-CDs in the inclusion complex. The intensity of UV-vis absorption
at a given wavelength will only depend on the amount of PAR monomers and PAR-(b-CD)n inclusion complexes
while b-CDs at the
mentioned wavelength were considered to have no absorption. Therefore, if n=1 in the
inclusion complex, the reaction in the solution is
H + G = HG (1)
where H is for b-CD, G for
PAR, and HG for PAR-b-CD
inclusion complex. According to reference [8], for the above reaction, a linear
relationship between [H]0[G]0/DA and [H]0 will be obtained if [H]0>>[G]0,
where [H]0 and [G]0 are the initial concentrations of host, b-CD and guest, PAR, respectively, DA is the difference of UV-vis
absorbance before and after the addition of b-CD. However, only a curve is obtained in our experiment (Fig.3),
which means that the assumption of 1:1 ratio of PAR to b-CD in the inclusion complex is not true.

Fig.3 The plot of [H]0[G]0/DA versus [H]0.
Similarly, the ratio of PAR to b-CD in the inclusion complex can be assumed as 1:2, that is
2H + G = GH2
(2)
The schematic structure of the inclusion complex, GH2 is shown as follows,

The dissociation constant, KD of
inclusion complex GH2 is defined as follows,
![]()
where x is the concentration of the inclusion
complex, GH2. The UV-vis absorbance, A, at a given wavelength (408 nm
used in this paper) can be described as follows,
![]()
where l is the path length, ![]() ,
, ![]() and
and ![]() are molar absorption coefficients of H,
G and GH2, respectively. In our experiment, [H]0>>[G]0.
Therefore, [H]0>>2x. Combining eq.3 and eq.4, we have
are molar absorption coefficients of H,
G and GH2, respectively. In our experiment, [H]0>>[G]0.
Therefore, [H]0>>2x. Combining eq.3 and eq.4, we have

Because there is no apparent absorbance of b-CD when wavelength ranges from 200 to
600nm, ![]() can be neglected comparing to
can be neglected comparing to ![]() and
and ![]() .
It is clear that DA=A-
.
It is clear that DA=A-![]() l[G]0. Let
l[G]0. Let ![]() , eq.5 can be rearranged as
, eq.5 can be rearranged as
![]()
The plot of [H]02[G]0/DA versus [H]02
is a straight line. That is the case for our experimental result shown in Fig.4.
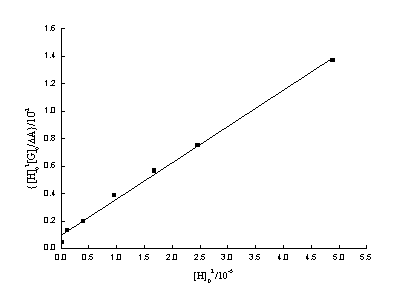
Fig.4 The plot of [H]02[G]0/DA versus [H]02.
A well behaved linear relationship with linear correlation coefficient r=0.998
between [H]02[G]0/DA and [H]02 confirmed that the
assumption of 1:2 ratio of PAR to b-CD in inclusion complex is correct. According to the slope and the
intercept of the line, the dissociation constant, KD, of the inclusion
complex is evaluated as 3.5¡Á10-6 mol·dm-3 and De is 3.9¡Á103 mol-1·
dm3· cm-1. Taking into account of the molar absorption coefficient of PAR(1.8¡Á104 mol-1· dm3·
cm-1), the molar absorption of the inclusion complex can be calculated as
1.4¡Á104 mol-1· dm3·
cm-1 at 408 nm.
3.2 Synthesis of inclusion complex by skiving
The inclusion complex of PAR and b-CD can be synthesized by sharable skiving[9]. PAR and b-CD with 1:2 ratio were mechanically
mixed to each other. The mixture was strongly skived for 3h under general humidity. Then
the mixture was rinsed with water and alcohol, respectively three times to remove the
monomers. The final product of the inclusion complex, a crystal powder with a light red
color, was obtained. The powder was dried overnight in a vacuum oven at 60ºC prior to use.
3.3 Infrared spectrum
Fig.5 shows the IR spectra of inclusion complex and both monomers. Fig.5a describes the IR
spectrum of b-CD. Fig.5b is
the IR spectrum of PAR. The IR spectrum of mechanical mixture of b-CD and PAR is shown in Fig.5c. In Fig.
5b, pyridyl group in PAR shows the characteristic absorption peaks at 1640, 1580 and
1430cm-1 which attributes to the stretching vibrations of pyridyl group.

Fig.5 IR spectra of b -CD (a), PAR(b), their
mixture(c),and the inclusion complex(d).
The results coincide with those reported by
reference [10]. All above absorption peaks can be found in Fig.5c, which
indicates that the absorption peaks of PAR are kept in the mechanical mixture. However,
from the IR spectrum of inclusion complex listed in Fig.5d, the original absorptions of PAR disappear comparing to Fig.5b and c but some new absorption
peaks occur which confirms that the formation of the inclusion complex because of the
interaction between b-CD
and PAR while PAR enters into the cavity of b-CD. PAR is incorporated within the hydrophobic cavity of b-CD, so the peaks in the donor's
infrared spectrum get broader and weaker. The combination of donor and acceptor is based
on the main binding forces including hydrogen bonding, Van der Waals forces, hydrophobic
interactions[11]. Because there is no chemical bond formed for this type of
inclusion complex, the shift of donor's absorption peak is not very striking[12].

Fig.6 Powder X-ray diffraction patterns of b-CD (a),PAR(b),their mixture(c),and the
inclusion complex(d).
3.4 Powder X-ray diffractometry
The formation of PAR-(b-CD)2
inclusion complex can be confirmed by the powder X-ray diffraction spectra. The X-ray
diffraction spectra of b-CD
monomer, PAR monomer, mechanical mixture of two monomers and the inclusion complex are
shown in Fig.6.
The X-ray spectrum of mechanical mixture, shown in Fig.6c, is also an
overlap of those of the host (Fig.6a) and guest (Fig.6b), while differently, the X-ray
spectrum of inclusion complex shown in Fig.6d is markedly different from Fig.6c. The X-ray
spectrum shows that the original crystal diffractions of PAR disappeared and PAR was
incorporated by b-CDs to
form new crystal diffractions[13]. Therefore, the inclusion complex is a new
substance which has different crystal structure comparing to the single donor and
acceptor.
3.5 Conclusions
An inclusion complex of b-CD
with PAR can be formed in aqueous solution and solid phase. The ratio of b-CD to PAR in the inclusion complex is
2:1. At 25ºC, the molar absorption coefficients of PAR and inclusion complex are 1.8¡Á104
and 1.4¡Á104 mol-1 ·dm3·
cm-1, respectively. The dissociation constant of the inclusion complex is
3.5¡Á10-6 mol·dm-3.
The authors also acknowledge the financial support from the Natural Science Foundation of Educational Committee of Jiangsu Province of China (Grant No. 03KJB150151), the Fund of Ministry of Education of China, and the Fund for an Academic Leader of QingLan Project of Institutions of Higher Learning in Jiangsu of China, which is a source for bringing up the future famous scientists.
REFERENCES
[1] Li S, Purdy W C. Chem. Rev., 1992, 92 (6): 1457.
[2] Connors K A. Chem. Rev. 1997,97 (5): 1325
[3] Nepogodiev S A, Stoddart J F. Chem. Rev., 1998, 98 (5): 1959.
[4] Murai S, Imajo S, Makietal Y. J. Colloid Interface Sci., 1996, 183 (1): 118.
[5] Sheremata T W, Hawari J. Environ. Sci. Technol., 2000, 34 (16): 3462.
[6] Tick G R, Lourenso F, Brusseau M L et al. Environ. Sci. Technol., 2003, 37 (24): 5829.
[7] The chemical reagents corporation of Beijing. Chemical Reagent·Fine Chemical
Product Handbookses(Huaxue Shiji Jingxi Huaxue Pin Shouce). Beijing: Chemical
Industry Press, 1999, 976.
[8] Zhang Yu, Diao GuoWang, Guo Rong. Journal of Yangzhou University (Yangzhou Daxue
Xuebao), 2002, 5 (3): 32.
[9] Tong LinHui. Cyclodextrin chemistry foundation and application (Huanhujing Huaxue
Jichu Yu Yingyong) . Beijing: Science Press,2001, 193.
[10] Standard Infrared Grating Spectra, USA: (Sadtler Research Laboratories, Inc.) 1980,
23-24: 23468k
[11] Stoddart J F, Zarzycki R. Recl Trav Chim Pays-Bas,1988,107 (9): 515.
[12] Colquhun H M, Stoddart J F, Williams D J. Angew. Chem., 1986,98 6): 483.
[13] Tong LinHui. Cyclodextrin chemistry foundation and application (Huanhujing Huaxue
Jichu Yu Yingyong). Beijing: Science Press, 2001, 211.
¡¡