http://www.chemistrymag.org/cji/2004/065038pe.htm |
May 2,
2004 Vol.6 No.5 P.38 Copyright |
He Xiaoqing1, Zeng Gucheng 1,
Cai Jiye1*, Wang Bin1, Feng
Qian1, WangChenxi2, Liang Zhihong3
(1Department of Chemistry, College of Life Science and Technology, 2Department
of Physics, College of Science and Engineering,3The Center for Experimental
Technology, Jinan University, Guangzhou 510632, China)
Supported by the National Natural Science Foundation of China ( No.30230350; No.60278014) and the National Key Research Foundation of China(973)(No.2001CB510101).
Abstract Chitosan has been widely used to remove the heavy metal ions such as Cu(II) and Ag(I) from waste solution. Self-organized nanostructure of chitosan induced by Cu(II) and Ag(I) on mica were imaged by atomic force microscopy, dendritic structures of chitosan which is fully self-simulated and tray-like feature of Cu(II)-Chitosan were obtained and compared to the one induced by Ag(I). Dendritic and tray-like features are very representative model of fractal including diffusion-limited aggregates model and Eden model. This work provides us an inspiration to identify the different chitosan possessing different chemical properties by topographic characterization. We infer that the fractal feature of chitosan brings the maximal capacity and less consumed cost to chitosan by increasing the linear measure when it is applied to absorb the heavy metal ions. These facts present that the fractal structure of chitosan gives another powerful example of the close relationship between function and its structure.Keywords Chitosan, Atomic force microscopy, fractal 1 INTRODUCTION
Chitosan, biodegradable and non-toxic[1] ,whose molecule has amino and hydroxyl groups which can be modified chemically[2], the free amine function of chitosan gives it a better ability to chelate ions of transition metals[3]which has been widely used in the removal of cations from waste solution[4] .However,most of current researches of the interactions between chitosan and heavy metal ions such as Cu(II) and Ag(I) which contaminated water seriously are based on SEM, X-ray, nuclear magnetic resonance(NMR) spectra, infrared spectra[5-11], Density functional theory(DFT)[12],and the actual understanding of how chitosan interact with heavy metal ions is not enough considering the key role of chitosan plays in pratical and theoretical field.
In this work,the self-organized nanostructure of chitosan induced by Cu(II), one of the most familiar contaminations were investigated by atomic force microscopy(AFM), a unique tool which is capable of monitoring the growth and transference of maromolecules such as chitosan at highy spatial resolution without damaging the properties of samples[13]. We also investigated the self-organized nanostructure of chitosan induced by Ag(I) parallelly, the results show the dendritic feature of chitosan and tray-like feature of Cu(II) or Ag(I)-Chitosan and the diameters of the "tray" of the chitosan induced by Cu(II) are larger than that of Ag(I).Furthermore, the growing models of chitosan and Cu(II) or Ag(I)- Chitosan obtained by AFM follows the fractal rules which is so pervasive in nature[14], this not only provides new evidence and inspiration to help understanding the fractal theory and probe the complex and mysterious fractal phenomenon that presents at nanometer scaled world but also obtain some useful evidences and novel angles to invertigate the interactions of chitosan with heavy metal ions due to their interesting and fascinating topography related to thier active fuction in practice. 2 EXPERIMENTAL SECTION
Chitosan was purchased from Shanghai Boao Biotechnology Company(Shanghai, China), the molecular weight is about 1.5¡Á106 , the intrinsic viscosity is less than 100cps,the degree of deacetylation is 90¨G.All chemicals are analytical grade and used without any further purification. 10 ml prepared chitosan and Cu(II) or Ag(I)-chitosan samples(pH 7.2) were deposited directly onto new freshly cleaved mica about 1.0¡Á1.0cm respectively (obtained from Sichuan Yaan Mica Industry Company, Sichuan ,China), Commercial silicon nitride (Si3N4)tips(Veeco,USA) and Non-contact model Autoprobe CP Research AFM (Thermomicroscope, USA) was used to perform the imaging at room temperature (25¡À1ºC) and air condition at a relative humidity of 80%. 3 RESULTS AND DISCUSSIONS
Firstly, we used FT-IR(data not shown here) to confirm that the Cu (II) and Ag(I)had binded to chitosan and Cu (II) or Ag(I)-chitosan existed on mica when they were added to the chitosan solution. For example, the peak of N-H£¨1633.69cm-1£©enhanced significantly when Cu (II) was added to the solution of chiotsan. As for the atomic force microscopy, we believed that the slower scan speeds will be better to probe the surface imformation though the facts demonstrated the surface feature shown below is not dependent on scan speeds.
3.1 Chitosan on mica investigated by AFM
As shown by the AFM images, Fig.1-A, prominent and delicate dendritic structures which is fully self-simulated were observed clearly. And all of these branches have the end, when two of these branches may encounter each other they will stop growing at the end of the branch though some branches composited of smaller particles go on walking towards triflingly. Fig.1-B shows the feature of growing clusters composed of some nanoparticles with varying values of the parameters about 20nm (Fig.1-C). In this work, all of the results were obtained at a fixed concentration of 0.1mg/mL of chitosan.We expected the construction of fractal topography of chitosan is independent of the concentration of the samples,however, the experiments were failed when at the concentrations of 0.01mg/mL or 1mg/mL It can be draw that the concentration of samples is crucial for the formation of the dendritic feature of chitosan .In addition,this beautiful delicate dendritic feature will remain persistent unless the surface is damaged deliberately.
3.2 Cu(II)-chitosan on mica investigated by AFM
Cu(II) solution with a concentration of 0.1mg/mL and 1mg/mL were respectively added to chitosan solution directly(1:1,V:V).Our experiment shows that the feature of Cu(II)-chitosan display a remarkably differently simple tray-like image(Fig.2-A), in contrast to the dendritic structure of pure chitosan. This fact suggests that the formation of Cu (II)-chitosan affect the unbiased random walk of chitosan in the grids on mica so that the feature of Cu (II)-chitosan alter significantly. This phenomenon will be more significant when the concentration of Cu(II) were increased to 1.0mg/mL. As shown by the Fig.2-B, more high density tray-like feature were observed and the diameter of the tray is almost at the same value about 35mm as the Cu(II)with a concentration of 0.1mg/mL. It indicates that more chitosan were induced to the tray by higher concentration of Cu(II).The simple tray-like or high density tray-like feature will disappear when the concentration of Cu(II) reduced to the value of 0.01mg/mL. And the prominency in the center of tray shown by the Fig.2-B and Fig.2-C demonstrate the fact that the clusters of Cu(II)-chitosan walk from periphery to the center with time and the clusters of Cu(II)-chitosan follow certain walking pathway to form the tray-like feature shown here . However, no tray-like feature will be observed if Cu(II) not reached the critical concentration, and we believe that higher concentration of Cu(II) will be favourable to observe the typical feature of Cu(II)-chitosan on mica.
3.3 Ag(I)-chitosan on mica investigated by AFM
It had been shown that the concentration of metal ion is crucial for the random walk behaviour of Cu(II)-chitosan clusters on mica. As it is shown by the Fig.4, we found that when Ag(I) with a concentration of 0.1mg/mL was added to the solution of chitosan(1:1,V:V), The tray-like feature of Ag(I)-chitosan on mica can also be observed repeatedly though the radius of tray decreased remarkably with a value about 4.49mm but the density of tray increased remarkably (Fig.3-A,B).The uniform appearance reflects a biquitous behaviour of Cu(II)-chitosan and Ag(I)-chitosan.
AFM provides a direct visualization of the dendritic and tray-like chitosan spontaneously self-organized on two-dimensional space of mica. For specific topography is substantially related to the inherent properties[15], an understanding of the affinity and mechanism of the interactions of Cu(II)-chitosan and Ag(I)-chitosan by topographic characterization manipulated by AFM has been of great interest. Futhermore, to demonstrate the reliability of the experiment , we repeated the experiments (Fig.2-D), the facts indicate that all the tray-like feature does not appear accidentally.
Dendritic and tray-like feature are very representative model of fractal including diffusion-limited aggregates (DLA) model[16] and Eden model [17] ,and these feature are observed persistently from pure chitosan to Cu(II)-chitosan and Ag(I)-chitosan, this fact reveals that the chitosan exhibits the fractal topography at large extension which are essential for its practical function. It is not an accident that chitosan exhibit the universal geometric derivation just as kinds of lives ,the fractal-like networks evolutived by natural selection effectively endow life with an additional fourth spatial dimension and maximize metabolic capacity and internal efficiency[18], ubiquity of fractal structures in nature provide perhaps the most convenient way to optimize the exchange of material, energy or information with the surrounding medium[19],fractal model endow chitosan a capacity of selecting a maximized efficiency to accomplish its function in the same way. We can infer that the fractal feature will bring the larger capacity and less consumed cost to chitosan when it is applied to remove the heavy metal ions because chitosan with fractal geometry will be equipped with infinite linear measure and increase the binding sites to metal ions significatively. It has been shown that the diameter of "tray" of Cu(II)-chitosan is larger than that of Ag(I)-chitosan. In the other words,the linear dimensions and volume of Cu(II)-chitosan is larger than the ones of Ag(I)-chitosan and this will increase the absorbing capability of chitosan for its exterior geometric shape. These facts present that the fractal structure of chitosan gives another powerful example of the close relationship between function and its structure and provides a fairly novel and useful angle to understand the complexity of the process of macromolecules such as chitosan from a solution towards a fractal surface feature even when they coexisted together with other molecules.
Some work had been performed by us suggest that the different topography will specialize specific chitosan, as shown above, the higher concentration of the ions the higher density of the "tray", and the value of diameter of the tray of Cu(II) is larger than the one of Ag(I),and this fact will drive us to identify the different chitosan possessing a different chemical properties though more extensive work should be carried out.
To our present work, it is hard to inspect the particular process of the chitosan particles growing on mica because the individual step will consume some time from one scan to another scan step to image by AFM. Determining the kinetics of the difference of fractal feature and the construction process of the fractal feature of chitosan and Cu(II) or Ag(I)-chitosan is not enough by the present work. Anyway, AFM provides a direct and real insights into fractal which is ubiquitous in the world by simple but powerful means comparing with such methods as computer simulation and complicated theoretical calculation.

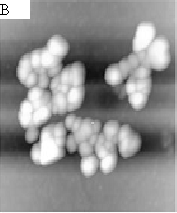

Fig.1 (A)representative AFM images of dendritic feature of chitosan on mica. Image size:30¡Á30mm,
(B) (C)are the images of clusters of chitosan , (B) 2.0¡Á2.0mm, (C)1.0¡Á1.0mm.



Fig.2 A typical AFM images of Cu(II)-chitosan on mica. (A) 35¡Á35
mm,(B) the tray image when Cu(II)at a concentration of 1.0mg/mL, 40¡Á40mm. (C)1.5¡Á1.5mm ,(C) allow us to see more details of the clusters of Cu(II)-chitosan moved from periphery towards the center with time, (D)repeated experiment , 30¡Á30mm.

Fig.3 AFM image of Ag(I)-chitosan on mica. image size: (A) 5.0¡Á5.0mm, the light area is as shown by the peak of the height profiles of (B). 4 CONCLUSION
The fractal feature of chitosan and self-organized Cu(II) or Ag(I)-Chitosan were investigated by atomic force microscopy. Dendritic feature of chitosan was observed while Cu(II) or Ag(I)-Chitosan show tray-like feature, and the higher concentration of the ions the higher density of the "tray", the diameter of "tray" of Cu(II)-chitosan is larger than that of Ag(I)-chitosan. This work will drive us to identify the different chitosan possessing a different chemical property. We also demonstrate that the fractal feature will bring the larger capacity and less consumed cost to chitosan when it is applied to remove the heavy metal ions by topographic characterization. The facts present that fractal structure of chitosan gives another powerful example of the close relationship between function and its structure. REFERENCES
[1] Muzzarelli R, Baklassare V et al. Biomaterials, 1988, 9: 247.
[2] In-Kyu Park, Jun Yang, Hwan-Jeong Jeong et al. Biomaterials, 2003, 24: 2331-2337.
[3] Muzzarelli R, Chitin.Oxford:Pergamon Press,1977.P.105.
[4] Piron E, Accorninotti M, Dornard A. Langmuir,1997, 13: 1653.
[5] Steenkamp G C, Keizer K, Neomagus J P et al. Journal of Membrane Science, 2002, 197: 147-156.
[6] Galo Ca'rdenas, Parra Orlando, Taboada Edelio. International Journal of Biological Macromolecules, 2001, 28: 167-174.
[7] Laurent Dambies, Claude Guimon, Sotira Yiacoumi et al. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2001, 177: 203-214.
[8] Fwu-Long Mi, Yu-Bey Wu, Shin-Shing Shyu et al. Journal of Membrane Science, 2003, 212: 237-254.
[9] Xianzeng Ye, Qinghua Yang, Yan Wang et al. Talanta, 1998, 47: 1099-1106.
[10] Kazuharu Yoshizuka , Zhengrong Lou, Katsutoshi Inoue. Reactive & Functional Polymers, 2000, 44: 47-54.
[11] Rodrigues C A, Laranjeira M C M, Stadler E et al. Carbohydrate Polymers, 2000, 42: 311-314.
[12] Braier N C, Jishi R A. Journal of Molecular Structure (Theochem), 2000, 499: 51-55.
[13] Cai J Y, Chen Y, Xu Q C et al. Molecules, 2003, 8: 86-91.
[14] Geoffrey B W, James H B, Brian J E. Science, 1997, 276: 122-126.
[15] Hazel Assender,Valery Bliznyuk, Kyriakos Porfyrakis. Science, 2002, 297: 973-976.
[16] Paul Meakin. Physica D, 1995, 86 :104-112.
[17] Thomas W H, Laura G, Steven G D et al. Physics Letters A, 1998, 250: 105-110.
[18] Geoffrey B W, James H B, Brian J E. Science, 1999, 284: 1677-1679.
[19] Helali N, Rezig B. Physica A, 2001, 292 (9): 25.
¡¡
¡¡