http://www.chemistrymag.org/cji/2004/069059ne.htm |
Sep. 1,
2004 Vol.6 No.9 P.59 Copyright |
Nucleation and growth of CaCO3 crystals on PDAC/PSS self-assembled multilayers
Du Zhuwei, Hao Juanling, Li Haoran( National Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100080, China)
Support from the National Natural Science Foundation of China (Grant 20306029).
Abstract Fabrication of biomimetic laminated composite by mimicking the cyclical matrix control processes to form the highly structured Mollusca nacre is presented in this paper. (PDAC/PSS)n self-assembled multilayers were built-up through alternative absorption of polycation and polyanion to a substrate as matrices and their chemical structure were characterized by UV-Vis absorption spectra. And then the deposition of CaCO3 took place on the multilayers by dipping them into a supersaturated CaCO3 solution. The morphology and the structure of the CaCO3 crystals were characterized by SEM and X ray diffraction respectively. With (PDAC/PSS)15 the hexagonal crystals of CaCO3 with the pellet size of 10-20
mm, which were very similar to the crystals existed in nacre, was obtained after 10 hr deposition.Keyword Self-assembled multilayer, Calcium carbonate, Biomimetic biomineralization, nacre
Natural biomineralization materials are
formed through the deposition of inorganic materials on organic matrices. The most
distinct feature of biomineralization processes is the formation of complex
inorganic-organic composite structures that are tailored to their respective function.
Nacre is a highly structured composite of calcium carbonate concentrated from seawater and
organic material secreted by mollusks. It is 1000 to 3000 times tougher than geological
aragonite[1,2]. The extremely high performance of nacre results from its
structure, which is governed by complex organism mediated mineralization processes[3].
Recently, the complicated mechanism of biomineralization is the subject of intensive
studies.
According to A. P. wheeler's work, mineralization cycle involves
selected release of matrix components and release of supersaturating levels of the ions or
matrix bond calcium carbonate. That is growth and cessation of growth alternate and the
matrix proteins regulate morphology and mineralogy of the growing crystals[4].
Generally, the organic compounds secreted by mollusks, which regulates crystal growth, are
polyanionic containing domains rich in aspartic acid and phosphoserice[5,6] and
ionic groups have been proven useful to investigate many aspects of biomineralization in vitro.
Thus we design an assembly route as shown in Scheme 1. Multilayer assemblies were
fabricated by consecutive adsorption of positive and negative charged polyelectrolytes[7,8].
It is relative easy to introduce different functional groups acting as mineralization
initiator compare to L/B films. The multilayers assembly is a similarity to the step of
the release of organic matrix and the crystal deposition is a simulate one to the bond of
calcium carbonate with the matrix. With these layer-by-layer assembled polymer films,
nacre biomineralization processes presented by Wheeler can be simulated.
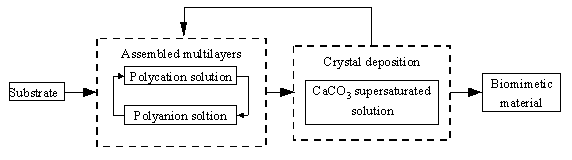
Scheme 1. General assembly route for biomimetic
laminated materials
Common and quartz glass substrates (thoroughly cleaned
before being used) were dipped into a solution of poly(diallyl-dimethylammonium chloride)
(PDAC, from Aldrich) of 0.01 monomol/L in Milli-Q water (monomol: mols of monomer repeat
units) for 15 min to form the first layer of PDAC. And then by immersed in a solution of
poly(styrenesulfonate sodium salt) (PSS, from Acros Organics) of 0.01 monomol/L in
purified water, formed a layer of PSS. Repetition of immersion of the substrate in the
above two solutions alternately should therefore lead to a multilayers (PDAC / PSS)n
assembly (n is the number of the bilayers). Driving force for the adsorption is
electrostatic attraction. The films exhibited typical ultraviolet absorption behavior of
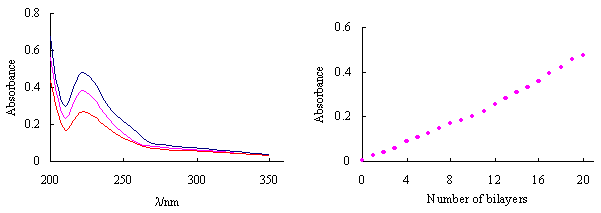
assembled multilayers. Figure 1-A shows the UV-vis absorbance spectra during the build-up
of a PDAC/PSS multilayer film. Absorbance intensity of multilayers at lmax(223nm),
which results from benzene ring in PSS, increased with the number of dipping cycles.
Figure 1-B shows a steady increase of thickness with the number of polymer layer, which
indicates the build-up of multilayers from the PDAC and PSS in a layer-by-layer manner. As
a result of the coulombic interaction nature for the multilayer formation, (PDAC/PSS)n
is believed to have a surface dominated by anions and (PDAC/PSS)nPDAC is to
have a surface dominated by cations. With (PDAC/PSS)n and (PDAC/PSS)nPDAC,
we can investigate the deposition process of calcium carbonate initiated by ¨C(CH2=CH-CH2)N+(CH3)2
and ¨CSO3- respectively.
(A)
(B)
Figure 1 UV-Vis spectra of PDAC/PSS multilayers varying with the number of bilayers
on quartz sheet. A: From bottom to top: 10, 15, 20 bialyers; B: The relationship of the
absorbance at 223nm vs. the number of bilayers
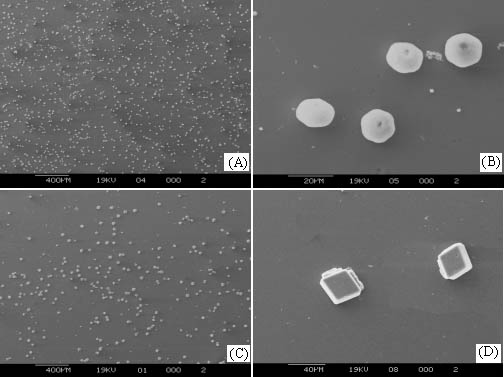
Figure 2 SEM images of CaCO3 crystals grown on the multilayers. A, B: (PDAC/PSS)15, crystallization time 10hr, C, D: (PDAC/PSS)15PDAC, crystallization time 10hr
Figure 2 shows the remarkable difference in morphology between the crystals grown on (PDAC/PSS)15PDAC and (PDAC/PSS)15. SEM micrograph of crystals grown on (PDAC/PSS)15 (Figure 2-A) has higher density and smaller size than that on (PDAC/PSS)15PDAC (Figure 2-C). Both samples show rather homogeneous size distribution. It is seen from Figure 2-B that hexagonal CaCO3 pallet crystals are on (PDAC/PSS)15 with a size of 10-20mm, which is very similar to the crystals existed in natural nacre. On the contrary, the morphology of the crystals regulated by ¨C(CH2=CH-CH2)N+(CH3)2 is quite different. Crystals on CaCO3 of (PDAC/PSS)15PDAC exhibits hexahedral shape and has a bigger size of about 30-40mm (Figure 2-D). Therefore, remarkable morphology similarity to natural biomineralization products could be obtained in the presence of artificial polyanion with ionic ¨CSO3- groups, though its chemical composition is by far different from anion groups of organics in nacre. However, X-ray diffraction results shown in Figure 3 indicate that crystals lattice of both the samples is different from any kind of natural nacre[9,10]. Consequently, we might suppose that electrostatic effect is the key factor in morphology control while the crystals lattice is determined by other factors.
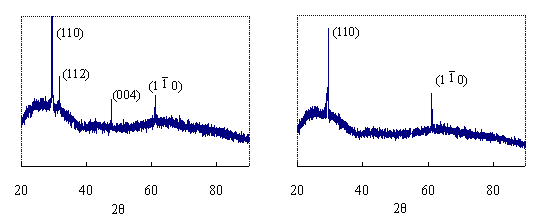
(A) (B)
Figure 3 XRD patterns of CaCO3 crystals regulated by the (PDAC/PSS)n multilayers. A: (PDAC/PSS)15, crystallization time 10hr, B: (PDAC/PSS)15 PDAC, crystallization time 10hr
REFERENCE
[1] Song F, Soh A K, Bai Y L. Biomaterials, 2003, 24: 3623-3631.
[2] Hou W T, Feng Q L. Journal of Crystal Growth, 2003, 258: 402-408.
[3] Su X, Belcher A M, Zaremba C M, et al. Chemistry of Materials, 2002, 14: 3106-3117.
[4] Wheeler A P, Sikes C S. Materials Research Society Symposium Proceedings, 2000, 599:
209-224.
[5] Graham T, Sarikaya M. Materials Science and Engineering: C, 2000, 11: 145-153.
[6] Grassmann O, Obmann P L. Biomaterials, 2004, 25: 277-282.
[7] Decher G. Science, 1997, 277: 1232-1237.
[8] Ladam G, Schaaf P, Decher G et al.. Biomolecular Engineering, 2002, 19: 273-280.
[9] Almqvist N, Thomson N H, Smith B L et al. Materials Science and Engineering C , 1999,
7: 37-43.
[10] Cui F Z, Feng Q L. Biomaterials, Beijing: Science China Press, 1996: 74-124.
¡¡