http://www.chemistrymag.org/cji/2004/06a064ne.htm |
Oct. 1,
2004 Vol.6 No.10 P.64 Copyright |
Zhang Haoqin, Liu Jindun
(School of Chemical Engineering£¬Zhengzhou University£¬Zhengzhou
450002)
Received on Apr.5, 2004; Supportted by Henan Innovation Project for University Prominent
Research Talents (2001KYCX006).
Abstract
The charge-mosaic membrane was prepared by interfacial polymerization for separating electrolytes from organics with low molecular weight. According to phenomenological equation and concentration polarization theory, a theoretical model describing membrane flux and membrane retention is established for electrolyte transport across the charge-mosaic membrane. The membrane parameters such as filtration coefficient, reflection coefficient and solute permeability are estimated. The results show that the model is in good agreement with the experimental data.Keywords Charge-mosaic membrane; electrolytes; transport mechanism
A charge-mosaic membrane consists of a set of anion and cation exchange elements regularly arranged in parallel and separated by the neutral matrix, each element providing a continuous pathway for the permeates from one bathing solution to the other. When pressure and concentration gradient are established across the membrane, the anions and the cations of electrolytes can flow in parallel through their respective pathways without a violation of macroscopic electroneutrality, resulting in a circulation of current between the individual ion-exchange elements. As a result, the charge-mosaic membrane shows the much greater permeability of salts than that of the non-electrolytes. In the recent twenty years, many fabrication methods such as polymer blending, graft co-polymerizing, and block co- polymerizing have been applied to prepare the charge-mosaic membrane [1]. However, the reports on the transport mechanism were few and the explanation of transport behaviors was basically in qualitative [2,3]. In the previous study [4], we have succeeded in fabricating the charge-mosaic membrane by using interfacial polymerization technique. For the practical use of charge-mosaic membrane, more detailed understanding about the membrane transport is still worth studying at present. For this purpose, the transport mechanism of electrolytes across a charge-mosaic membrane will be discussed in this paper. The membrane parameters such as filtration coefficient, reflection coefficient and solute permeability are determined.
1 THEORY
1.1 Concentration polarization
Usually, the apparent retention Ra is used to express the separating
capability of a membrane, that is:
![]() (1)
(1)
Here, Cb and Cp represent the feed concentration
and permeate concentration, respectively, mol/L.
For the separation process by using charge mosaic membrane, the concentration at the membrane surface Cm is greater than Cb owing to the
influence of concentration polarization. The real retention Rs can be defined
as follows:
![]() £¨2£©
£¨2£©
According to the concentration
polarization theory , the following equation can be obtained:
![]() £¨3£©
£¨3£©
Where Jv is membrane flux,
m3/(m2·s1)£»k is mass transfer coefficient, m/s.
1.2 Phenomenological equation
For the charge-mosaic membrane with high quality, the anion and cation exchange materials
are highly permselective. Since none of the practical applications envisaged for the
mosaic involve the passage of net current across the membrane, we need only to concern
with the two-process phenomenological equations for salts and volume flow. According to
the analysis by Weinstein[5], volume flux and salt flux, Jv and Js, are
expressed by the following equations, respectively, related to the driving forces
hydrostatic pressure ![]() , osmotic pressure
, osmotic pressure ![]() , and chemical potential difference
, and chemical potential difference ![]() :
:
![]() £¨4£©
£¨4£©
![]() £¨5£©
£¨5£©
Where Lp is the filtration coefficient,
s is the reflection
coefficient, w is the solute permeability, and Cs is the average
concentration.
Considering that the retention of salt is lower for the charge-mosaic
membrane, the average concentration of salt can be adopted as the arithmetic average concentration instead of logarithmic
average concentration. When hydrostatic pressure DP>>Dp, we suppose ![]() . Thus the following equation can be obtained by substituting Eq. (4) and
(5) into Eq. (2) :
. Thus the following equation can be obtained by substituting Eq. (4) and
(5) into Eq. (2) :
 £¨6£©
£¨6£©
Where ![]() ;
T is temperature, K; R=8.314 J/(mol·K). KS is the ions
transported by the membrane, which is based on the type of salts.( For NaCl£¬KS=2£»Na2SO4
and MgCl2£¬KS=3)
;
T is temperature, K; R=8.314 J/(mol·K). KS is the ions
transported by the membrane, which is based on the type of salts.( For NaCl£¬KS=2£»Na2SO4
and MgCl2£¬KS=3)

Fig. 1 Scheme of the experimental apparatus
1.feed reservoir 2.pump 3.manometer 4.membrane module 5.tank for permeation
2 EXPERIMENTAL
The charge-mosaic membrane was prepared by using interfacial polymerization technique.
Polyethersulfone (PES) hollow fiber membrane was used as the support membrane. Firstly,
the aqueous solution containing 2, 5-diaminobenzene sulfonic acid and polyethylenimine
(PEI) was introduced into the fiber from the lumen and kept for about 30 minutes.
Different additives and/or acid acceptors were also added into the aqueous amine solution.
Then, the dodecane solution containing trimesoyl choride acid (TMC) and 4-(chloromethyl)
benzoyl chloride was gently fed into the fiber for the interfacial polymerization
reaction. After the polymer layer was formed, the membrane was immersed with an aqueous
trimethylamine solution and kept for 24 hours to convert the chloromethylated groups into
cationic quaternary ammonium groups. The membrane finally was immersed in distilled water
until use in the test.
The membrane separating experiments were carried with NaSO4
and MgCl2. The initial concentration of salts was 0.01 mol/L. The
concentrations of the permeated salts were measured with microprocessor conductivity
meter. Fig.1 is the experimental scheme for measuring the volume flow and the
apparent retention of salts.
3 RESULTS AND DISCUSSION
3.1 Calculation of the real retention
Because both the diameter and the length of hollow fiber membrane are very small, the
fluid flowing through the fiber belongs to laminar flow and the laminar boundary layer is
not fully developed. The mass transfer coefficient can be calculated[6]by
Eq.(7):
![]() £¨7£©
£¨7£©
Where, d is the diameter of hollow fiber,
m; L is the length of hollow fiber, m; Re is Reynolds number; Sc is Schmidt number [![]() ]; D is diffusivity and can be calculated
according to the ion solution chemistry theory[7] for electrolytes, m2/s.
]; D is diffusivity and can be calculated
according to the ion solution chemistry theory[7] for electrolytes, m2/s.
Once the mass transfer coefficient is obtained, the real
retention will be determined by equation (3) and (2). Under the experimental conditions,
the real retention is higher than the apparent retention by 5-10%.
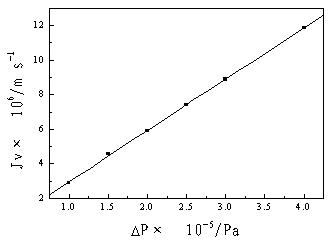 |
Figure 2 The filtration coefficient |
3.2 Model Parameter Fitting
The filtration coefficient can be obtained by fitting the experimental data of water flux
in Figure 2, that is:
Lp= 2.976¡Á10-11 m3/(m2·s1·Pa1
)
The correlation coefficient R is 0.9998.
According
to Eq. (6), the reflection coefficient and the solute permeability can be calculated based
on nonlinear curve fitting to the experimental data of Na2SO4 and
MgCl2 by using Origin software [8]:
for Na2SO4£¬s=0.46 £¬w=3.93¡Á10-10
mol/(N1·s1);
for MgCl2£¬s=0.37£¬w=5.177¡Á10-10 mol/(N1·s1).
Figure 3 shows the
comparison of theoretical values with experimental data, in which the solid lines are the
theoretical values calculated by equation (6) and the dispersing dots are the experimental
data. It shows that the theoretical values fit well with the experimental
data.
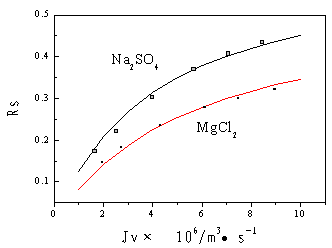 |
Figure 3 Comparison of the theoretical values with experimental data |
4 CONCLUSIONS
The transport mechanism of electrolytes across a charge-mosaic membrane is discussed. The
membrane coefficients can be determined by fitting the experimental data. The mathematical
model is in good agreement with the experimental data.
REFERENCES
[1] Zhang H Q, Liu J D, Fan G D. J. of East China University of Science and Technology
(Huadong Ligongdaxue Xuebao), 2003, 29 (6): 569-574.
[2] Ishizu K, Amemiya M. J. Membr. Sci., 1992,65 (1-2): 129-140.
[3] Yamauchi A, Tateyama J., Etoh B I et al., J.
Membr. Sci.,2000,17 (2): 275-280.
[4] Liu J D, Zhang H Q. The 2nd International
Conference on Application of Membrane Technology, Beijing :The membrane industry
association of china, 2002, 268-271.
[5] Weinstein J N, B. Bunow J, Caplan S R. Desalination,1972,
11: 341-377.
[6] Mulder M . (translated by Li L). Basic Principles of Membrane Technology (Mojishu
Jiben Yuanli), 2nd edition, Beijing: Tsinghua University Publisher, 1996:270-275.
[7] Liu G, Qiu Z H. Ion Solution Chemistry (Lizi Rongye Huaxue), Fuzhou: Fu-jian
Technology Publisher, 1987: 251-275.
[8] Hao H W, Shi G K. Origin 6.0 Professional (Origin 6.0 Shili Jiaocheng), Beijing: China
Electric Power Publisher, 2000: 88-104.