http://www.chemistrymag.org/cji/2004/069063ne.htm |
Sep. 16,
2004 Vol.6 No.9 P.63 Copyright |
Zhou Guobina,b, Zhang Pengfeia, Chen Zhenchub
, Pan Yuanjiangb
(aDepartment of Chemistry Hangzhou Teachers College, Hangzhou 310036; bDepartment
of Chemistry Zhejiang University , Hangzhou 310027, China)
Received on Apr.5, 2004; Supportted by the National Natural Science Foundation of China (No 203760160) and the Natural Science Foundation of Zhejiang Province (No 202075)
Abstract Selenazole derivatives have antitumor, antibacterial and other notable activities. The crystal structure of 5-acetyl-2,4-diphenylselenazole that we report here displays the molecular configuration and provides an important evidence for the mechanism of cyclocondensation of a-tosyloxylketones with selenoamidesKeywords Mechanism, Structure Characterization, 5-acetyl-2, 4-diphenylselenazole
The selenazole derivatives are of marked interest because of their antitumor, antibacterial and other notable activities[1-3]. The author's research group have reported a new effective method for synthesis of selenazoles (scheme 1) by cyclocondensation of
a-tosyloxylketones with selenoamides in one pot without use of lachrymatory and toxica-haloketones[4]
Scheme 1 Table.1 The crystal data and structure refinement for 5-acetyl-2,4-diphenylselenazole
Empirical formula |
C34H26O2N2Se2 |
Z value |
2 |
Formula weight |
652.51 |
F(000) |
656.00 |
Crystal color,Habit |
colorless, prismatic |
Diffractometer |
Rigaku AFC7R |
Crystal Dimensions |
0.20¡Á0.20¡Á0.30mm |
Radiation, graphite |
MoKa |
Crystal system |
triclinic |
Refinement |
Full-matrix least-squares |
Lattice Type |
primitive |
Goodness-of-fit indicator |
2.48 |
Space group |
P1(#2) |
Residual:R; Rw |
0.043, 0.058 |
a(Å) |
10.516(3) |
Largest difference peak |
0.87, -0.71e-1/ Å3 |
b (Å) |
14.224(7) |
No. of Reflections Used for Unit |
¡¡ |
c (Å) |
10.232(4) |
Cell
Determination |
23(18.4¡ª25.7¡ã) |
v (Å3) |
1420(1) |
¡¡ | ¡¡ |
In our experiment, we
think that this kind of cyclocondensation reaction may be carried out following two kinds
of mechanisms (scheme 2) and get isomeric compound. In order to investigate the mechanism
of this kind of reaction, we choose the 5-acetyl-2,4-diphenylselenazole as typical example for crystal
X-ray. 
Scheme 2
The single crystal x-ray structure (Fig.1.) shows that the skeleton contains five membered selenazole ring, with two benzyl groups attached to the atoms C(1)and C(2) and acetyl group attached to the atom C(3). The benzyl ring attached to the atoms C(1) is almost parallel to the plane defined by (C1, C2, C3, N1, Se1), the corresponding torsion angle is -10.06¡ã. Due to the space obstacle, the benzyl ring attached to the atoms C(2) deviate much to the plane of selenazole ring, the corresponding torsion angle is 57.39¡ã.
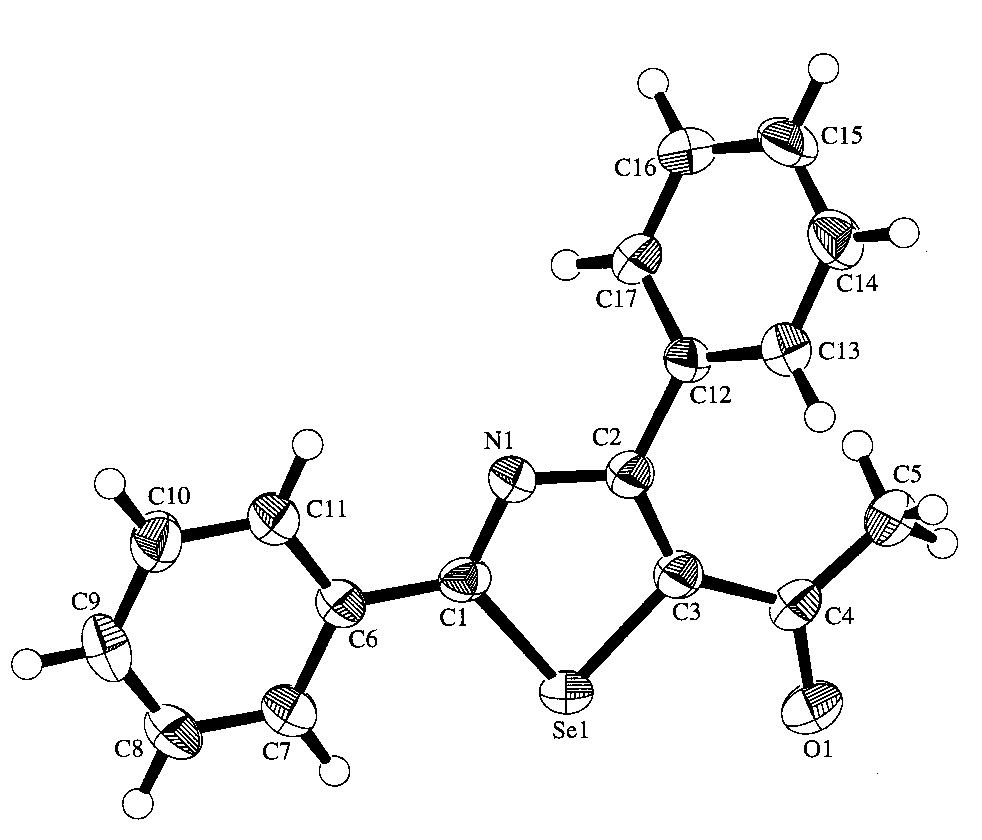
Fig .1 The molecular structure of 5-acetyl-2, 4-diphenylselenazole
In conclusion, The crystal structure that we report here displays the molecular configuration of 5-acetyl-2,4-diphenylselenazole and provides a important evidence for the Mechanism I of the cyclocondensation of a-tosyloxylketones with selenoamides. *Crystallographic data for the structural analysis have been deposited with the Cambridge Crystallographic Data Center, CCDC reference the number 234082. Copies of this information may be obtained free of charge from: The Director, CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK(fax: +44-1223-336033; E-mail: [email protected] or http://www.ccdc.cam.ac.uk). REFERENCES
[1] Shafiee A , Mazloumi A and Cohen V I. J. Heterocycl. Chem, 1979, 16: 1563.
[2] Shafiee A , Shafaati A and Khamench B H. J. Heterocycl. Chem., 1989, 26:709.
[3] Shafiee A, Khashayarmanesh Z and Kamal F. J. Sci. Isamic.Repub.Iran, 1990, 1: 111.
[4] Zhang P F, Chen Z C. Synthesis, 2000, 9: 1219-122.
[5] Sheldrick G M, Kruger C, Goddaed R SHELXS86, OxfordUniversity, Press, 1985, 175-189.
[6] Molecular Sturucture Corporatoion. Crystal Structure Analysis Pagckage ---- TeXsan, 1985 and 1992.
[7] Beurskens P T, Admiraal G, Beurskens G et al. DIRDIF92, 1992.
[8] Cromer D T, Wabwe J T. International Tables for X-ray Crystallography (Vol. IV). Birmingham: The Kynoch Press, Table 2.2 A, 1974. ¡¡