http://www.chemistrymag.org/cji/2004/06c097ne.htm |
Dec. 1,
2004 Vol.6 No.12 P.97 Copyright |
Zhang Junling, Zheng Wenjie, Zou Jiahao, Yang
Fang, Bai Yan, Li Yiqun
(Department of Chemistry, Jinan University, Guangzhou 510632 China)
Supported by the National Natural Science Foundation of China(No.20271022) and Guangdong
Natural Science Foundation (No.010369).
Abstract
A series of heterocyclic aromatic selenium compounds were synthesized at room temperature in solid-state with ortho-aromatic diamines and selenium dioxide. The yields of the synthesized compounds are as follows: 2,1,3-benzoselena-diazole(BS)77.1%; 1,2,5-selenadiazolo- [3,4-b]pyridine(SPb)43.8%;1,2,5-selenadiazolo[3,4-c]pyridine(SPc)22.9%;5-methyl-2,1,3-benzo-[3,4-c]selenadiazole(MB)73.5%;1,2,5-selenadiazole[3,4-d]pyrimidine-7-(5H,6H)-dione(SPO)50.5%; 5,7-dihydroxy-1,2,5-selenadiazolo-[3,4-d]pyrimidine (DHSP)18.9% and 2,1,3-naphtho-[2,3-c]- selenadiazole (NS) 76.7% . The products prepared by the solid state reaction were characterized by IR, XRD, EA, ICP and compared with the authentic sample obtained from liquid state reaction. The results showed that the reaction under solid state condition was benign to the environment, completed with higher yields and more convenient work-up.Keywords Heterocyclic aromatic selenium compounds; selenadiazole; solid state synthesis
Selenium is the trace element controlled by gene in human body[1]. Heterocyclic aromatic selenium compounds have many unique properties, such as biological activities of antivirus[3] and superconductivity. They are very useful in many fields and also very important intermediates in organic synthesis[3]. Solid phase synthesis has become an important protocol for medicine and new functional because of the advantages of easily controlling, high yields, friendly to the environment , easily removing of the excess reagents and soluble byproducts[4]. The reaction of ortho-aromatic diamines and selenium dioxide is a complex process including a series of steps such as nucleophilic (electrophilic) attack, ring-closure and elimination. Accordingly, investigation of the reaction producing selenadiazoles by solid phase method at room temperature attract much attention. To continue our previous work in solid phase synthesis, herein we wish to report the further study on the application of solid-state synthesis in synthesizing heterocyclic aromatic selenium compounds.
1 EXPERIMENTAL
All ortho-aromatic diamines were
purchased from Sigma and used without further purification, other reagents were commercial
available. The water was distilled twice in the quartz distiller before use. The
analytical instruments were Perkin-Elmer Optime 2000 DV inductively coupled plasm,
ELEMENTAR Vario element analysis, Bruker Equinox 55 infrared spectrum and XD-98 X-ray
diffraction.
Diamines and selenious oxide were ground respectively, and then were
mixed at the ratio of 1:1 in a mortar at room temperature, the process were monitored with
XRD or IR. During the process, apparent phenomena were observed. The results showed that
the reactions were completed after 30 minutes of grinding and the desired products were
obtained. The crude products of BS、MB、NS were dissolved in cyclohexane, and filtrated. The filtrate were
washed three times with doubly distilled water, after evaporating of the cyclohexane, the
corresponding products are practically pure without further purification. The compounds of
SPO and DHSP were added enough doubly distilled water, mixed entirely, and recrystallized
according to the reference [5]. As for the crude product of SPb, dissolved with benzene,
filtrated and the filtrate was vaporized, the residue was dissolved in the mixed solvent
of ether-acetone (1:1), filtered off the active carbon after decoloration, and remove the
solvent to obtain the target product. As about the SPc, dissolved with ether, then
filtrated and add the solvent of ether from petroleum into it , rubbed the wall of the
beaker, vaporized the solvents and obtained the target product. The pure products were
stored in dark place. The reactants, crude products were characterized by IR, XRD, and the pure products were analyzed with ICP and EA.
The liquid state synthesis and purification of the compound of SPO
according to the literature [5], BS, MB, NS and DHSP according to the literature [6], SPb according to
literature [7].
2 RESULTS
The reaction process were monitored conveniently by X-ray diffraction and IR. Take BS
as an example, some results were showed in Fig.1. It can be seen that the signals of
reactants such as diamines and selenious oxide were disappeared in the chart of the target
product, the signals of the crude products were resembled with the purified product and
indicated the reaction was completed and the purity of the crude product was high. The
product obtained from solid-state reaction (SSR) was in accord with that from liquid-state
synthesis (LSR), so they were the same compounds. The data of IR were showed in Table 2.
Table 1 summarized the data of element analysis. The experimental data
were in agreement with the calculated value, indicating that the products obtained from
solid-state reaction were the expected compounds.
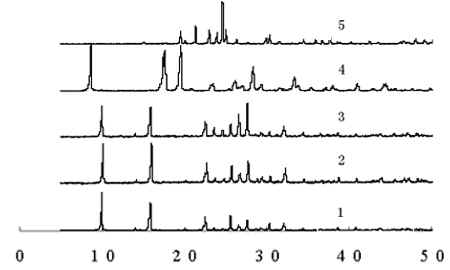
Fig. 1 The XRD chart of
BS and its reactants through different synthesis methods
1: BS obtained from LSR; 2: BS obtained from
SSR; 3:crude product from SSR; 4: 1,2-phenylenediamine, 5:SeO2
Table 1 The Element Analysis data of the compounds
Compounds |
The experimental data (calculated value)% |
|||
Se1) |
C |
H |
N |
|
BS |
43.20(43.13) |
39.46(39.36) |
2.32(2.20) |
15.44(15.30) |
MB |
39.83(40.06) |
43.10(42.66) |
2.99(3.07) |
14.34(14.21) |
NS |
34.03(33.87) |
50.36(51.52) |
2.60(2.59) |
11.59(12.02) |
SPb |
42.67(42.52) |
33.12(32.63) |
1.80(1.64) |
22.12(22.83) |
SPc |
42.67(42.31) |
33.86(32.63) |
2.10(1.64) |
22.66(22.83) |
SPO |
39.00(39.27) |
24.01(23.90) |
1.18(1.00) |
28.06(27.87) |
DHSP |
36.38(36.35) |
21.99 (22.15) |
1.10 (0.93) |
25.40 (25.81) |
SpectralStrip distribution [8-10] |
BS |
MB |
NS |
SPb |
SPc |
SPO |
DHSP |
| n(CH) +t(R) +n(nh) | 3038 |
3047 |
3040 |
3049 |
3073 |
3176 |
3056 |
n (C=O) |
|
|
|
|
|
1701 |
1704 |
| t(R) | 1497 |
1497 |
1488 |
1500 |
1510 |
1460 |
|
| g(C-N) | 1466 |
1445 |
1377 |
1434 |
1405 |
1384 |
|
1346 |
|
1346 |
|
1356 |
|
|
|
1132 |
1149 |
1141 |
1123 |
1163 |
1124 |
|
|
| t(R)+g(C-H)+b(R) | |
|
854 |
916 |
906 |
910 |
|
| n(N-Se-N)+ n(C-C) | 709 |
710 |
737 |
711 |
|
|
|
| n(N-Se-N) | 489 |
500 |
468 |
495 |
491 |
447 |
495 |
The yields of the
heterocyclic aromatic selenium compounds prepared by different synthetic methods were
showed in Table 3. It showed that the yields under solid-state reaction were higher than
that under liquid-state synthesis.
Table 3 The yields of heterocyclic aromatic selenium compounds obtained
by different synthetic methods
selenium compounds |
BS |
MB |
NS |
SPO |
SPb |
SPc |
DHSP |
solid-state |
77.1% |
73.5% |
76.7% |
50.5% |
43.8% |
22.9% |
18.9% |
liquid-state |
72.1% |
70.5% |
70.7% |
40.5% |
38.3% |
11%1) |
10.0% |
The study of Tod illuminated that the solid state reaction carried out at room temperature with high selectivity and yields[4]. The reaction of diamines and selenious oxide is called "Hinsberg Reaction", namely the classical method to synthesize this kind of compounds , and also one of the most important techniques to synthesize organic selenisume. It can be expressed as follows:
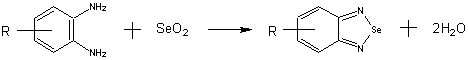
The reaction discussed in this paper was a complex process including a series of steps such as nucleophilic (electrophilic) attack, ring-closure and elimination and so on. The results showed that the heterocyclic aromatic selenium compounds could be synthesized by the solid-state reaction at room temperature, and the yields were higher than the corresponding reactions in liquid-state. Traditionally, this kind of reaction was carried out in the solution conditions of ethanol or a dilute acid existing. The compounds have two nitrogen atoms which can be easily protonized in dilute acid, this byproducts bring some drawbacks such as the separation and purification. However, the solid-state reactions avoid the formation of the protonized byproducts because of the only byproduct water, which can be easily removed. This is the main reason of the high yields of "Hinsberg Reaction". Presumably, the solid-state synthesis of heterocyclic aromatic selenium compounds has great theoretical and applicable values in the fields of organic synthesis.
REFERENCES
[1] Rotruck J T. Science, 1973, 179: 588-590.
[2] Zhang J L, Zou J H, Zheng W J et al. Chemical Research and Application (Huaxue Yanjiu
YuYingyong), 2004, 16 (4): 561-562.
[3] Spiros Grivas.Current Organic Chemistry, 2000, 4: 707-726.
[4] Zhou Y M, Xin X Q. Chinese Journal of Inorganic chemistry (Wuji Huaxue Xuebao), 1999,
15 (3): 273-292.
[5] Alvert Carr, Eugene Sawicki, Francis E Ray. J. Org. Chem, 1958, 23: 1940-1943.
[6] Zheng W J, Zeng X H, Yang F, et al. Chinese Journal of analytical science (Fenxi Kexue
Xuebao), 2003, 19 (1): 36-38.
[7] Brown N M D, Peter B. Tetrahedron, 1968, 24: 6577-6582.
[8] Kwiatkowski J S, Leszczynski J, Teca I. Journal of molecular structure, 1997, 436-437:
451-480.
[9] Liu Q F, Zhang D T, Wang X L. Chinese Journal of Spectroscopy Laboratory (Guangpu
Shiyanshi), 2003, 20 (6): 845-847.
[10] Written by Pretsch E, Buhlmanm P, translated by Rong G B. Structure determination of
organic compounds tables of spectral data. Shanghai: East China University of Science and
Technology Press, 2002.