http://www.chemistrymag.org/cji/2005/071001pe.htm |
Jan.1, 2005 Vol.7 No.1 P.1 Copyright |
Preparation of dendritic silver nanoparticles by direct chemical reduction
Li Guoping, Luo Yunjun, Yao Weishang, Zhu
Liang ,Tan Huimin
(School of Materials Science and Engineering, Beijing Institute of Technology, Beijing
100081)
Received on Oct. 22, 2004; Supported by the Trans-century Training Programme Foundational for Talents by the State Education Commission.
Abstract Preparation of dendritic silver particles by direct chemical reduction of silver ions under the protection of PVP was studied in this work. Effects of molar ratio of PVP to silver ions and the reaction time on the morphology and structure of silver particles were also described. When PVP/AgNO3=1 and the reaction time was about 1h, the well-defined dendritic silver particles were achieved. The mechanism of dendritic silver nanoparticle has been proposed. Firstly, the complex of PVP-silver ions is constructed. Secondly, the complex promotes silver nucleations. Thirdly, aggregation and limited dispersion leads to formation of dendritic silver nanoparticle.Keywords Dendritic silver nanoparticles, PVP, Direct chemical reducation
1 INTRODUCTION
In recent years, noble metal particles have been widely studied for their excellent
properties [1,2], and their potential applications in microelectronics,
optical, electronic, magnetic devices, and catalyst [2-4]. As we know, the
intrinsic properties of a noble metal nanoparticle strongly depended on its morphology and
structure. Silver nanoparticles with different shapes has attracted a lot of interest
because silver exhibits the highest electrical and thermal conductivities among all
metals. Since dendritic structure has many special characteristics, such as its large
surface area, well conductivity etc. and the ability to provide a natural framework for
the study of disordered system, dendritic nanostructure of noble metal is an attractive
supramolecular structure. Recently, various methods have been developed for preparing
dendritic noble metal nanoparticles, such as template-based method [5], gas
evaporation method [6], and electrochemical deposition [7].
Dendritic silver nanoparticles were observed by ultraviolet irradiation photoreduction
technique, or by sonoelectrochemical methods, or under microwave irradiation [8].
Zhu et al. used nickel nanotubes as reducing agent through a hydrothermal method to
prepare dendritic silver, and discussed the important role of nickel nanotubes for
dendritic growth [9].
Herein, the study of formation of dendritic silver with PVP (poly
(N-vinly-2-pyrrolidione)) as the stabilizer and ethanol as reducing agent has been
reported. The condition and apparatus of this method are mild. It is focused on the effect
of PVP/ AgNO3 mole ration on the morphology and structure of the dendritic
silver. Based on TEM and XRD, the mechanism for forming the dendritic silver will be
proposed.
2 EXPERIMENTAL
All chemicals were of analytical grade and were used without further purification. Silver
nitrate, ethanol were purchased from Chemical Reagent Company, Beijing, China. PVP (K30)
was imported from Fluka Chemie. The above materials were dissolved in deionized water to
form aqueous solution.
In a typical process, 10 ml aqueous solution of PVP (0.1 mol/L)
(pH=5.18) was added into a 100ml three-necked flask which was equipped with a condenser,
magnetic stirring bar, and thermcontroller, then 5 ml aqueous solution of AgNO3
(0.1 mol/L) was added by syringe when the solution was being stirred. After 10 minutes,
5ml ethanol was added drop by drop by syringe and the mixed
solution was stirred .The reaction solution was kept under nitrogen and at 80℃ until the reduction was complete.
UV-Vis absorption was recorded with a Unico UV2102PC UV-Vis
spectrophotometer. Transmission electron microscopy and electron diffraction image of
product were taken with JEM-2010 apparatus. Samples for them were prepared by dropping
product onto carbon-covered cooper net, and the solvent was evaporated naturally. FT-IR
spectra were observed with NEXUS-470 FTIR (Nicolet) and X-ray powder (XRD) analysis was
performed on a Japan Rigaku diffractometer with Cu as target.
3 RESULTS AND DISCUSSION
3.1 Influence of PVP/ AgNO3 mole ratio on the size, morphology of product
Varying amounts of the protective agent PVP were used for investigating the effect of
the polymer on silver particles size and shape. Fig.1 was the UV-Vis absorption spectrum
of the prepared silver nanoparticles. The absorption peak centered at about 420 nm is the
characteristic peak for silver particles and is attributed to the plasmon absorption band
of silver particles. With an increase in the ratio of PVP/AgNO3 (one mole of
PVP means one unit of PVP), the intensity and the maximum wavelength of the peak
increased. The large change in the optical spectra implied that the dendritic silver
transform to the sphere-like silver nanoparticles.
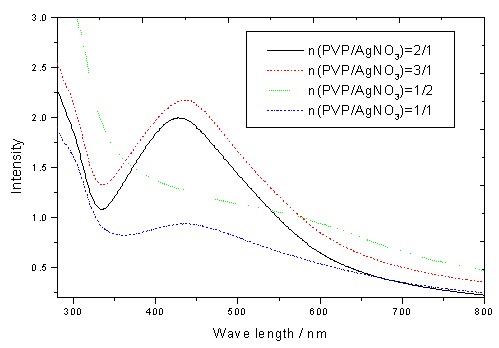
Fig. 1 UV-vis spectra of mixed solution
containing 5ml (0.1M) AgNO3 and 5ml(0.1M) PVP after reduction by methanol for
1h
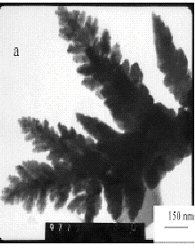
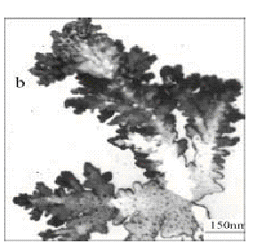
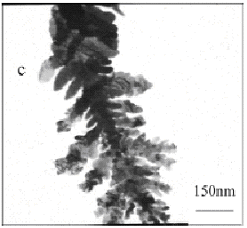
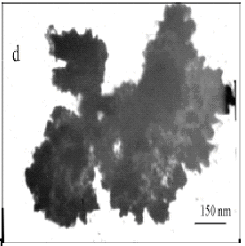
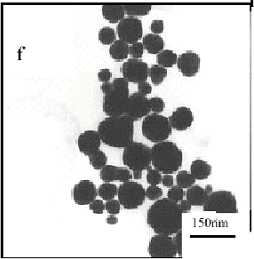
Fig. 2 TEM micrographs of silver particles at the mole
ratios of (a) PVP/AgNO3=1, (b) PVP/AgNO3=2, (c) PVP/AgNO3=0.5,
(d) PVP/AgNO3=0, and (f) PVP/AgNO3=3.
Fig.2 showed TEM micrographs of the obtained silver particles. When
PVP/AgNO3=0, it revealed irregular shape and
sinter-like particles with several micrometers in size (in Fig.2d). The well-defined
nanoscale dendritic silver particles with pine-like shape were obtained in the case of
PVP/AgNO3=0.5, 1, and 2 (Fig.2c, a, b). But when PVP/AgNO3=1, the as-prepared particles showed symmetric branched dendritic structures
(Fig.2a). Fig.2c showed that the dendritic silver particles grown from a big cluster in
the case of PVP/AgNO3=0.5, and these some particles with several nanometers in
size were seen in Fig.2b (PVP/AgNO3=2) and the color of the dendritic silver
particles were paler. When PVP/AgNO3=3, the well-dispersed sphere-like
particles were obtained (Fig.2f). This means that the particles shape and size distinctly
depend on different PVP/AgNO3 mole ratios and PVP/AgNO3=1 is a
better ratio for preparing the dendritic silver nanoparticles. Hence the PVP/AgNO3=1
will be used in the next experiment.
Fig.3 was the typical XRD of as-prepared dendritic silver particles
(PVP/AgNO3=1), in which four strong peaks correspond to the (111), (200), (220)
and (311) of face-centered cubic (fcc) Ag (JCPDS No.4-0783).
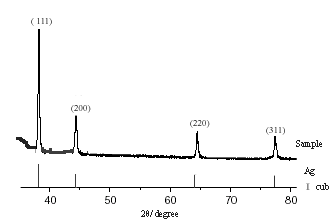
Fig.3 XRD of the dendritic silver particles (PVP/AgNo3=1)
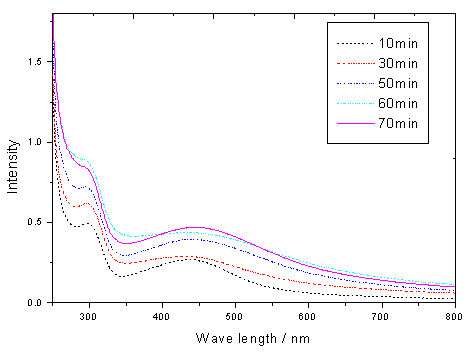
Fig.4 The change of the UV-vis absorption spectra
of the system as the function of time
3.2 Influence of the reaction time on morphology of product
The stoichiometric reaction between ethanol and silver ion in aqueous solution can be
written as follows:
4Ag+ + CH3CH2OH + 5OH-
-----> 4Ag + CH3COO- +4 H2O
So, if only ethanol was used, the reduction rate would be slow at room
temperature due to low pH (pH=5.18). However the low reduction rate was so necessary to
prepare the dendritic silver that had enough time for forming dendritic silver
nanoparticles. Therefore the morphology of silver particles was strongly dependent on the
reaction time. The intensity of the peaks around 430nm, which is assigned to the plasma
resonance of Ag particles, increased and the peak shifted to the longer wavelength (red
shift) with an increase of the reaction time (shown in Fig.4), showing an increase in the
size of the silver nanoparticles. But when the reaction time exceeded over 70min, the
intensity reduced by contraries. The effect may be explained by the changing size and
morphology of silver particles. Indeed, in the present method, the well-dispersed
sphere-like silver nanoparticles were obtained at early times (Fig.5a), because at this
stage, little silver ions were reduced into atoms as a result of lower reducing ability of
methanol and correspondingly, the amount of PVP was sufficient for surrounding the silver
particles and preventing the obtained particles from agglomeration. The inset SAED pattern
of silver nanoparticles indicates that these nanoparticles are single-crystallized with
fcc structure. When the reaction time was prolonged to 60min, the well-defined dendritic
silver particles were achieved (Fig.5c). But when the reaction time was prolonged, some
precipitations appeared in the reaction solution of which the TEM image was shown in
Fig.5d owing to the PVP protection effect being not complete. Moreover, Voigt et al. [10]
reported that the dendritic pattern was formed in non-equilibrium growth, so when the
reaction system gradually transformed from non-equilibrium to equilibrium with an increase
of the reaction time, the dendritic pattern disappeared.
3.3 Mechanism of PVP protection and formation of dendritic silver nanoparticles
PVP(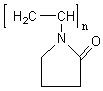 )has a structure of a polyvinyl skeleton with polar groups. The
donated lone pairs of both nitrogen and oxygen atoms in the polar groups of one PVP unit
may occupy two sp orbital of the silver ions to form a complex compound. We suppose that this is the first step of the dendrtic silver
formation, which may be identified by detecting the precipitation while changing the
adding order of PVP and methanol, or by comparing the absorption spectrum of the AgNO3
solution containing PVP or not containing PVP. It is noted that pure PVP has no absorption
peak in ultraviolet spectra[2]. The exhibit of the solution containing AgNO3
at 323nm resulted from the coordinative bonds of H2O:Ag:OH2. At the
same Ag + concentration, the addition of PVP to the AgNO3 solution
produced a new band at 430nm assigned to well-known d-d transition for Ag+ of
the complex PVP-Ag+, which showed that the nitrogen and oxygen of the PVP have
a stronger coordinative field than H2O.
)has a structure of a polyvinyl skeleton with polar groups. The
donated lone pairs of both nitrogen and oxygen atoms in the polar groups of one PVP unit
may occupy two sp orbital of the silver ions to form a complex compound. We suppose that this is the first step of the dendrtic silver
formation, which may be identified by detecting the precipitation while changing the
adding order of PVP and methanol, or by comparing the absorption spectrum of the AgNO3
solution containing PVP or not containing PVP. It is noted that pure PVP has no absorption
peak in ultraviolet spectra[2]. The exhibit of the solution containing AgNO3
at 323nm resulted from the coordinative bonds of H2O:Ag:OH2. At the
same Ag + concentration, the addition of PVP to the AgNO3 solution
produced a new band at 430nm assigned to well-known d-d transition for Ag+ of
the complex PVP-Ag+, which showed that the nitrogen and oxygen of the PVP have
a stronger coordinative field than H2O.

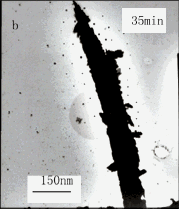

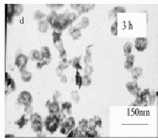
Fig.5 Effect of reaction time on TEM images of silver
particles
The subsequent step is
PVP-accelerated formation of a large amount of silver nuclei. It was found that the color
of the solution changed gradually from pink at the initial reaction to violet at the end
of the reaction. TEM observation showed that a large amount of small silver nuclei at the
beginning of the reductive reaction were produced (in Fig. 5a).
The third step is the formation of dendritic sliver particles. There is
no doubt that PVP is a kind of polymer to prevent the silver particles from aggregation
according to previous works [2]. So on the basis of PVP prohibiting particles
aggregation, we can explain that well-dispersed sphere-like particles were produced in
solution of PVP/AgNO3=3 (Fig. 2f) and the more strongly aggregated silver
particles (Fig. 2d) are due to lack of protective by PVP. Although the prohibiting effect
of PVP has been seen in solutions of PVP/AgNO3=0.5, 1, or 2, partial
agglomeration also takes place as a result of incomplete covering of silver particles by
PVP. Then new silver particles are produced on the surface of agglomeration and PVP may
play a role of making silver particles limited dispersion. The two processes are carried
out by turns as reductive reaction goes on, which at last
leads to the formation of dendritic silver particles.
In addition, an aqueous solution of dendritic silver particles stored
for over 2 months at room temperature remained stable. The fine stability of the
nanocomposite solution was due to PVP as protective agent for Ag particles.
4 CONCLUSION
The well-defined nanoscale dendritic silver particles
with pine-like shape has been prepared by reducing silver nitrate with methanol in the
presence of PVP as protective agent.
It was found that the molar ratio between PVP and silver ions and the
reaction time could play very important roles in determining the silver morphology, size
and its stability.
The mechanism of dendritic silver nanoparticle has been proposed.
Firstly, the complex of PVP-silver ions is constructed. Secondly, the complex promotes
silver nucleations. Thirdly, aggregation and limited dispersion leads to formation of
dendritic silver nanoparticle.
REFERENCE
[1] Chen S W, Huang K, Stearns J A. Chem. Mater., 2000, 12: 540.
[2] Zhang Z, Zhao B, Hu L. J. Solid. State Chem., 1996, 121: 105.
[3] Chou K S, Ren C Y. Mater. Chem. and Phy., 2000, 64: 241.
[4] Colvin V L, Schlamp M C, Alivisatos A P. Nature, 1994, 370: 354.
[5] Xiao J, Xie Y, Tang R et al. Adv. Mater., 2001,13: 1887.
[6] Gao Y, Jiang P, Liu D et al. Chem. Phys. Lett., 2003, 380: 146.
[7] Wang S, Xin H. J. Phys. Chem. B, 2000, 104: 681.
[8] Liao X, Zhu J, Qiu X et al. J. Nanjin University (Natural Science) (Nanjing Daxue
Xuebao), 2002, 38: 119.
[9] Zhu Y, Zheng H, Li Y, et al. Mater. Res. Bull., 2003, 38 : 1829.
[10] Tian Z, Liu J, Voigt J A et al. Nano Lett., 2003, 3: 89.
直接还原法合成树枝状银纳米颗粒
李国平,罗运军,姚唯尚,朱亮,谭惠民
(北京理工大学材料科学与工程学院,北京,100081)
摘要 采用PVP为稳定剂,通过直接还原法合成了树枝状银颗粒。研究银粒子与PVP摩尔比以及反应时间对对银纳米颗粒的形状的影响,探索形成树枝状银颗粒的机理。
关键词 树枝状银,PVP,直接还原法