http://www.chemistrymag.org/cji/2005/071003pe.htm |
Jan.21, 2005 Vol.7 No.1 P.3 Copyright |
(College of Resource and Environmental Science; #Center of Analysis and Testment of Wuhan University, 430072 Wuhan, China) Received on Dec. 13, 2004; Support by the National Natural Science Foundation of China (20277028).
Abstract A novel method for the separation
and preconcentration of platinum with crosslinked chitosan (CCTS) and determination by
graphite furnace atomic spectrometry (GFAAS) has been developed. CCTS was synthesized by
reacting chitosan with epoxy chloropropane. The adsorption rate of CCTS for Pt (IV) was
100% at pH 3-4 when the adsorption time was not less than 20min. Pt (IV) was eluted off
from CCTS with 5 mL mixture of 0.1 M HCl and 3% thiourea and determined by GFAAS. The
detection limit (3s, n=6)
for Pt (IV) was 0.43ng /mL and the relative standard deviation was less than 8.4 % . The
method was applied to environmental water samples with recoveries of 90-94%. Moreover, the
adsorption mechanism of CCTS for Pt (IV) was discussed.
Keywords adsorption, Crosslinked chitosan, Graphite furnace atomic spectrometry,
determination, platinum
1 INTRODUCTION
The platinum is mainly used in automobile exhaust catalytic converters and used as a
catalyst in a wide variety of processes such as nitric acid production and petroleum
reforming. Also, some platinum co-ordination compounds are used as anti-cancer drugs in
medical treatment. Soluble platinum compounds are toxic and chronic, industrial exposure
to them is responsible for the syndrome called platinosis. The small particles for the
abrasion of the catalytic surface can penetrate deeply in human lungs and are
toxicological relevant [1,2]. Moreover, they are able to form various
organo-metallic compounds. In these forms they can be solubilised and enter soils,
sediments, waters and plants, and in consequence, they can enter food chain [3].
The determination of platinum metals is becoming of increasing interest in medical and
environmental samples.
Various methods have been developed for platinum separation and
preconcentration from environmental and biological matrices. Bosch et al. described
on-line preconcentration of platinum on a column packed with silica gel functionalized
with 1,5-bis (di-2-pyridyl) methylene thiocarbohydrazide (DPTH-gel) placed in the
autosampler arm and determinated by electrothermal atomic absorption spectrometry [4].
Lásztity described a flow-injection graphite furnace atomic absorption
spectrometric method. This method was worked out by using oxime, sulphoxine and
2,2-diamino-diethylamine (DEN) cellulose microcolumns for preconcentration of platinum
after reduction by iodide or sulphite ions [5]. Kubrakova et al. [6]
applied preconcentration of low levels of platinum group metals on a polystyrenebase
adsorbent containing diethylene triamine groups. The concentrated metals were determined
in slurries by Zeeman ET-AAS.
Chitosan (CTS) is a deacetylation product of chitin and can effectively
remove toxic metals in waste because of its strong adsorption [7]. However, CTS
can form salt and be dissolved in acid media because the free amino group (-NH2)
in CTS is protonized (-NH3+), which is a disadvantage in
application. Crosslinked chitosan (CCTS), synthesized by reacting chitosan with epoxy
chloropropane, can keep its rigidity in acidic solution and can retain the good adsorption
properties for many metal ions. CCTS was used to separate and preconcentrate many metal
ions, such as Mn (VII)/Mn (II),Cr (III)/Cr (VI)[8,9].
In this work, Pt (IV) was preconcentrated by CCTS and determined by
GFAAS, the possibility of application of this procedure was studied for analysis of
environmental waters.
2.1 Apparatus
AAS measurements were carried with a Hitachi 180-80 spectrometer. A platinum hollow cathode lamp (Hitachi, Japan) was used as a light source. The graphite furnace instrumental parameters of determination and temperature-time program of atomization are listed in Table 1 and Table 2 respectively. The pH values were measured with a Delta 320-s pH meter (Mettler-Toledo, Greifensee, Shanghai, China). A magnetic stirrer (Nanhui Telecommunications Equipment Factory ,Shanghai,China) and a HY-8 speed control oscillator(Guohua Instrument Factory,Jiangshu,China) were applied. Reagents were weighted by AY-120 electric balance (SHIMADZU Corporation, Japan). The IR spectra of CCTS before and after adsorption were obtained by a Model 170-SX infrared spectrometer (Nicolet, Madison, WI, USA). Table 1 Instrumental parameters of determination
| Parameter | Pt |
| Wavelength (nm) | 265.9 |
| Bandpass (nm) | 0.4 |
| Lamp current (mA) | 12.5 |
| Sample volume (μl) | 10 |
Step |
Temperature/℃ |
Ramp time/s |
Hold time/s |
Argon flow /mL min-1 |
Dry |
80-120 |
20 |
10 |
200 |
Ash |
800 |
20 |
10 |
200 |
Atomization |
2600 |
0 |
5.0 |
0 |
Clean |
2800 |
0 |
3.0 |
200 |
Throughout the analysis, distilled water was applied. All reagents were of analytical grade. Platinum stock solution (1mg /mL) was prepared by dissolution of 1g of Chloroplatinum acid in 94.1 mL aqua regia. The stock solution stored in glass bottles in refrigerator and working solutions were obtained by dilution. 3% thiourea was prepared by dissolving 3g of thiourea reagent in 100mL 0.1M HCl.
2.3 Synthesis of the crosslinked chitosan (CCTS)
Firstly, chitosan was obtained from raw materials of chitin using deacetylizing reactions. Then, the product (6.0 g) was dissolved in 320 mL of 1% (m/v) acetic acid. To this, 6 mL of epoxy chloropropane was slowly added, vigorously stirring, followed by 50 mL of 5% (m/v) NaOH added in drops. After reacting for about 18 h at room temperature, a white solid was precipitated. The resulting precipitate was filtered off, washed thoroughly with distilled water and a little propanol, and dried in vacuo. After panmilling and sieving (200 mesh), it was ready for use in the experiments.
2.4 Procedures
A water sample was filtered with a membrane of 0.45 mm. The pH value of a 500 mL water sample was adjusted to 3. The solution was then transferred to a flask containing 50 mg CCTS, vibrated for 20 min at room temperature and filtered. The Pt (IV) remaining on the CCTS was eluted with 5mL mixture of 0.1 M HCL and 3% thiourea ,washed with deionized water and diluted to 10 mL ,then determined by GFAAS. 3 RESULTS AND DISSCUSSION
3.1 Effect of pH on platinum adsorption
0.5mL of 0.1 mg/mL Pt (IV) was added to a beaker of 50 mL and adjusted to desirable pH value by 0.1 mol/L HCl or NaOH . The final solution volume should not be exceeded 40 mL. The solution was then transferred to a flask containing 20 mg CCTS and shaken for 20 min at room temperature. The filtrated solution was diluted to 50 mL with distilled water and determined by GFAAS. Fig. 1 shows the effect of pH on the adsorption rate for 50mL standard solutions of 1mg/mL Pt (IV). The optimum pH value was between 3-4.
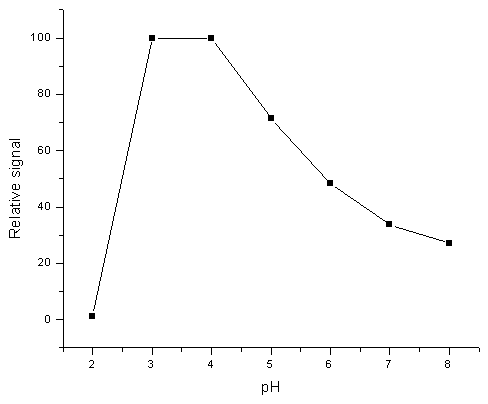
Fig.1 Effect of pH on platinum adsorption
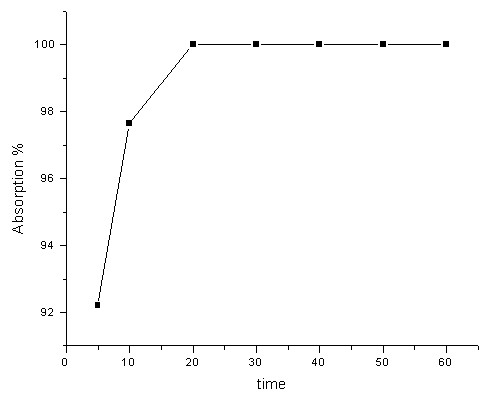
Fig. 2 Effect of preconcentration time 3.2 Effect of preconcentration time
Fig. 2 shows the effect of adsorption time on the adsorption of Pt (IV), 20 mg of CCTS was used. The adsorption rate reached a maximum value at 20 min.
3.3 Effect of CCTS dosage
For the determination of 50 mL of 1mg/mL Pt (IV) at different CCTS dosage ,the preconcentration efficiency reached a maximum value when CCTS dosage was not less than 20mg.
3.4 Effect of sample solution volume
The sample volume had an effect on the preconcentration efficiency even though the amount of CCTS added was enough to adsorb the total amount of Pt from the aqueous samples. Experiments were conducted using 50 mg CCTS to preconcentrate 0.25mg Pt (IV) in different volumes of the solution. The filtrated solution was determined by GFAAS. Table 3 shows that when 50 mg CCTS was used, the sample solution volume up to 500 mL did not affect the adsorption of Pt (IV). Therefore, a sample solution volume of 500 mL was used in the preconcentration of Pt (IV). Table 3 Effect of sample volume
Sample solution volume (mL) |
CCTS dosage (mg) |
Pt (IV) ( mg) |
Adsorption rate (%) |
100 |
50 |
0.25 |
100 |
200 |
50 |
0.25 |
100 |
300 |
50 |
0.25 |
100 |
400 |
50 |
0.25 |
100 |
500 |
50 |
0.25 |
100 |
A series of Pt (IV) standard solutions were analyzed using standard procedures , the results are shown in Fig. 3. It could be seen that the saturation capacity of adsorption of CCTS to Pt (IV) was 725.75mg/g.
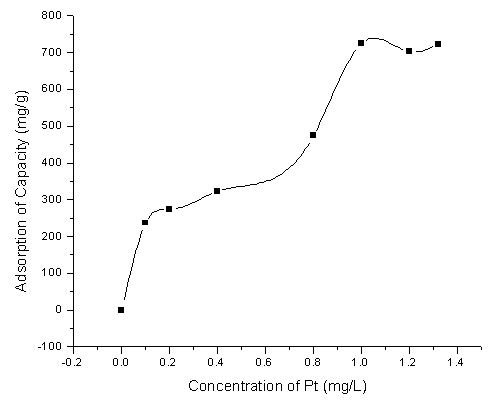
Fig.3 Adsorption Capacity of CCTS for Pt (IV) 3.6 Effects of foreign ions
The effects of some foreign ions on the preconcentration and determination of 500 mL of solution containing 5mg Pt were tested. No interferences were observed when Na+(5.38g), K+(190mg), Mg2+(640mg), Ca2+(200mg), Al3+(234mg), Br-(13.4mg) Cl-(9.55g), Ag+ (100mg), NO3-(62.0mg), SO42-(1.33g) ,Ni2+(64.43g) were present, Pd2+(45mg) had little effect to adsorption of Pt (IV), but had negative effect on the elution of Pt (IV).
3.7 Determination of performance of the method
According to the above mentioned procedure, 500mL of 10ng/mL Pt (IV) standard solution were measured after adsorption by CCTS. The detection limit (3s, n=6) for Pt (IV) was 0.43ng/mL and the relative standard deviation less than 8.4%.
3.8 Analysis of real samples
The results obtained from water samples are listed in Table 4. Because no standard reference materials of water were available, the accuracy of the methods was controlled by spiking the samples. Table 4 Analysis of water samples
| Samples | Pt (IV) added (mg) | Pt (IV) found (mg) | Recovery (%) |
Lake water |
0.00 | 0.00 | — |
| 10 | 9.32 | 93.2 | |
Sea water |
0.00 | 0.00 | — |
| 10 | 9.13 | 91.3 | |
Waste water |
0.00 | 0.00 | — |
| 10 | 9.08 | 90.8 |
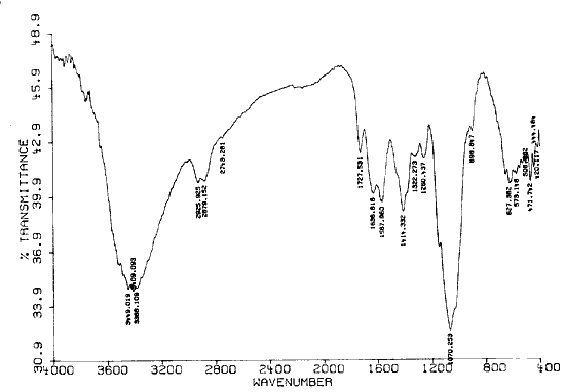
Fig.4 The FT-IR spectrum of CCTS before adsorption
4 MECHANISM OF ADSORPTION OF CCTS
In acid media, the free amino group (-NH2) in CCTS is protonized (-NH3+). The reaction is the following equation:
CCTS- NH2+H2O → CCTS-NH3+ +OH-
According to the static gravitation, CCTS with positive charge can adsorb anions of charge neutralization exists in acid solution in anion group forms (PtCl4-), hence Pt (IV) can be strongly adsorbed by CCTS. Fig.4 and Fig.5 show the FT-IR spectrum of CCTS before and after adsorption for Pt (IV) respectively. The characteristic absorption peaks of amino and hydroxyl is at 3387 cm-1, carbonyl of amide is at 1662 cm-1, and secondary alcohol and primary alcohols are at 1067 and 1025 cm-1 respectively. Compared with the IR spectrum of CCTS before adsorption, it can be seen that the main adsorption peaks (-NH2 and -OH) of CCTS have not changed. The results indicate that the action between CCTS and Pt (IV) is mainly physical adsorption.
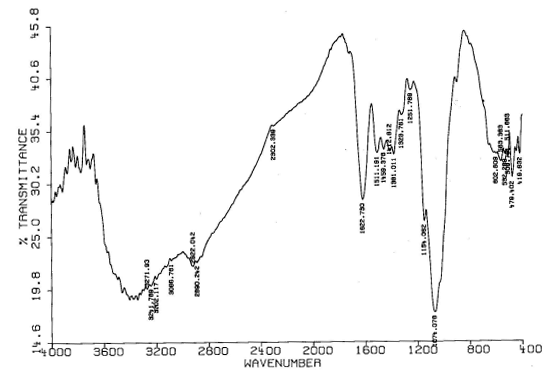
Fig.5 The FT-IR spectrum of CCTS adsorbed Pt (IV)
5 CONCLUSION
Through the research, we can see that this method is suitable for the separation, preconcentration and determination of Pt (IV) in environmental water samples because of its high sensitivity, low detect limit and analytical cost.
REFERENCES
[1] Kanitsar K, Koellensperger G, Hann S et al. J. Anal. At. Spectrom, 2003, 18: 239.
[2] Ch Wei, Morrison G M. Anal. Chim. Acta, 1994, 284: 587.
[3] Farago M E , Kavanag P, Blanks R et al. J. Anal.Chem, 1998,123: 451.
[4] Bosch Ojeda C , Sánchez Rojas F , Cano Pavón J M , Garcya de Torres A .
Analytica Chimica Acta , 2003, 494: 97.
[5] Lásztity A , Kelkó-Lévai A , Zih-Perényi K, Varga I.
Talanta, 2003, 59: 393.
[6] Kubrakova I V , Kudinova T F , Kuzmin N M et al. Anal. Chim. Acta , 1996, 343: 167.
[7] Yan J . Chin. Chem. Bull, 1984, 11: 26.
[8]
[9] Jiang J S , Huang G Q , Qian S H , Wang Y T. Environ. Sci., 1997, 18: 69.
交联壳聚糖预富集石墨炉原子吸收分光光度法测定环境水样中痕量铂
汪光1 钱沙华1 黄淦泉1 罗燕 1 莫少波2
(武汉大学资源与环境科学学院; 武汉大学分析测试中心 430072 武汉市)
2004年12月13日收稿; 国家自然科学基金项目(20277028)
摘要 本文报道了用交联壳聚糖预富集分离、石墨炉原子吸收分光光度法测定环境水样中痕量铂的方法。交联壳聚糖由壳聚糖与环氧氯丙烷交联反应而成。在pH值为3-4,吸附时间大于或等于20分钟时,对铂的吸附率可达到100%。用5mL0.1mol/L HCL-3%柠檬酸溶液将被交联壳聚糖吸附的铂洗脱下来,然后用石墨炉原子吸收分光光度计测定。该法的检出限为0.43ng/mL,相对标准偏差小于8.4%。用于环境水样中痕量铂的测定,回收率为90-94% 。本文还探讨了交联壳聚糖对于铂的吸附机理。
关键词 交联壳聚糖、吸附、石墨炉原子吸收、分析、铂