http://www.chemistrymag.org/cji/2005/071005pe.htm |
Jan.21, 2005 Vol.7 No.1 P.5 Copyright |
Study on the cyclic voltammetric behavior of the chloroaquorhodium(III) complexes
Gu Yongwana* , Dong Shouan b, Zhang Huiyingc, Duan Deliangd, Wang Shixingb(aKunming Sino-Platinum Metals Catalyst Co.,Ltd, Kunming 650101; bKunming Institute of Precious Metals, Kunming 650221; cDepartment of geoscience, Kunming University of Science and Technology, Kunming 650093; dDepartment of Chemistry, Peking University, Beijing 100871, China)
Received on Aug. 8, 2004.
Abstract
The cyclic voltammetric behaviors of the chloroaquo complexes of rhodium(III ) on glassy carbon electrode were explored. Under various condition of pH value, potential range, scanning rate and concentration of rhodium( III) , a number of cyclic voltammograms of hexaaquorhodium(III ) ions in sodium perchlorate medium are attained. The results indicate that the optimized conditions include pH=2, 20ug/ml Rh(III ) , 1 mol·dm-3 sodium perchlorate and a scanning rate of 70 mv/sec. At the same time, a rapid irreversible three electrons reduction produces at about -0.2v. The asymmetric curve of this process is due to the diffusion controlled mass transfer. In addition, there are some other factors that can affect the shape of curve, such as the adsorption, instability of the oxidized or reduced species, etc. The peak height does not depend on the concentration of rhodium(III ) in the solution, for this reason, this cyclic voltammetric response is difficult to be used to determine rhodium(III ) content. With regard to other chloroaquorhodium(III ) complexes, apart from the [Rh(H2O)Cl5]2- and [RhCl6]3+ species, two waves are present in the process of reduction, the former is adsorption wave, the latter is the three electrons reduction wave.Keywords Chloroaquorhodium(III ) Complexes; Cyclic Voltammetry; Electroanalysis There have been various early papers concerned with the electrochemical behavior of the chloroaquorhodium(III) complexes, especially for their polarographic and coulometric behavior. Generally, the rhodium(III ) complexes, e.g., the halo compounds, yield a polarographic wave with two or more steps, for which the total limiting current is proportional to the rhodium(III) concentration[1]. The electron exchange number of the total process is 3. However, in the cases of fluoride solution and pyridine and g -picoline complexes[2], Rh(III) and Rh(III) appear as relatively stable intermediate states in the reduction process. Kravtsov and Shereshevskaya[3] assume that the first wave of the reduction polarogram of rhodium(III) chloro complex corresponds to the step Rh(III)→Rh(III). The polarographic results suggest that except for [RhBr6]3-[4], all the rhodium complexes behavior appear irreversibly.
The reduction of [Rh(H2O)6]3+ in 0.13 mol·dm-3 (HClO4 + NaClO4) solutions at a DME(dropping mercury electrode) was studied by Van Loon[1]. The polarograms exhibit two waves, the first of which was irreversible and diffusion controlled with E1/2=-0.38v. The second wave showed a marked maximum that did not change notably by addition to solution of maximum suppressors such as gelation or methyl red. The polarography of haloaquo complexes of rhodium(III ) was studied by Cozzi and Pantani[4,5] as a function of complex concentration and pH value of the solution. For each species, the polarograms showed two steps of different height and required the addition of gelation to the solution in order to obtain well-defined waves. The limiting current of the first step was lower than that of the second step and decreased with increasing concentration of rhodium(III). The total limiting current of both waves is diffusion controlled. According to these authors, the first wave would correspond to the reduction of Hg22+ formed by spontaneous oxidation of the DME by the rhodium(III) complexes. The second wave would be rhodium amalgam. In every case, the half-wave potential becomes more negative as the concentration of chloride ion increases. This fact suggests the intermediate in the process of several types of complex with a different number of chloride ligands.
The research by Van Loon and Page[1] on the reduction of rhodium(III) in 0.1 mol·dm-3 (HCl + NaCl) solutions with a DME also yielded polarograms with two waves. The first wave is composite and its total limiting current depends on the rhodium concentration, the second wave is very similar to that for the rhodium-aquo complex and shows a maximum that is not controlled by a diffusion process. Kravtsov and Shereshevskava[3] investigated the polarographic waves of rhodium(III) in 4 mol·dm-3 (HCl + HClO4) solution. At low rhodium concentrations (3×10-5 mol·dm-3), the first wave corresponds to the reversible reduction process Rh(III) + 2e « Rh(III) i.e., to RhCl2-. The second wave, which is irreversible and diffusion controlled, is ascribed by the authors to reduction of the RhCl4- ion. This complex is supposed to be in equilibrium with the species RhCl52- and RhCl63-, which are the predominant species in the solution.
Chemical and electrochemical studies aimed at obtaining rhodium(III) coordination complexes with more familiar ligands have been unsuccessful[6]. The oscillographic polarography and the catalytic waves of rhodium(III) complexes were studied in detail by Beran and Dolezal[7], Douglas and Magee[8], Gao Xiaoxia and Zheng Jianbin[9,10]. Although the polarographic, oscillographic and coulometric behaviors have been studied in many papers, no systematic investigation has been carried out into the cyclic voltammetric behavior of the chloroaquo complexes of rhodium(III).
2. EXPERIMENTAL
2.1 Apparatus and Reagents
All measurements were performed with BAS 100B/W electrochemical analyzer (Bioanalytical
systems, Inc) in a three-compartment cell with a glassy carbon working electrode, platinum
wire auxiliary electrode and silver/silver chloride reference electrode. The pH value was
measured with PHs-3 pH meter. High-purity nitrogen was used for deaeration. All
chloroaquorhodium(III) complexes used in experimental are made by the author's samples laboratory[11]. The samples were stored at 40C
in a refrigerator. Redistilled water is used. All other chemicals are analytical reagent
grade.
2.2. Experimental Procedure
2.2.1 Selection of working electrode
Different supporting electrolytes (0.1, 0.5, 1, 2 mol·dm-3 NaClO4
and 0.1, 0.5, 1, 2 mol·dm-3 HClO4, respectively) were used.
The cyclic voltammograms were recorded from +0.4 to -1.0v
(vs.Ag/AgCl) via utilizing the glassy carbon electrode and platinum electrode as working
electrode respectively. The scanning rate is 100 mv/s and sensitivity is 100 uA/v. The two
series of cyclic voltammograms are compared and the ideal working electrode is selected.
2.2.2 Selection of acidity of supporting electrolytes containing rhodium(III)
1 ml [Rh(H2O)6]·(ClO4)3 solution
containing 200 ug/ml of rhodium(III ) was added to 9 ml of 1 mol·dm-3-
NaClO4 solution and five sample solutions were prepared similarly. The pH value
of solution was adjusted to 2, 3, 4, 5, 6, 7 with perchloric acid and sodium hydroxide
respectively. Before the measurements, the solution must be deaerated with high purity
nitrogen for 5 minutes. Using glassy carbon electrode as working electrode(result attained
from section 2.2.1), the cyclic voltammograms of various solutions were recorded from +0.4
to -0.8v (vs. Ag/AgCl). The scanning rate is 100 mv/s
and sensitivity is 100 uA/v. According to the current response (i.e. peak height) and
other conditions, the optimum pH value is selected.
2.2.3 Selection of scanning rate
1 ml [Rh(H2O)6]·(ClO4)3 solution
containing 100 ug/mL of rhodium(III ) was added to 9 mL of 1 mol·dm-3
NaClO4 solution. The pH value of solution was adjusted to 2 with perchloric
acid and sodium hydroxide. Using glassy carbon electrode as working electrode, the cyclic
voltammograms at different scanning rate (60, 70, 80, 90, 100, 110,120 mv/s respectively)
were recorded from +0.4 to –0.8 v (vs. Ag/AgCl). The
sensitivity is 100 uA/v. According to their peak shape, the optimum scanning rate is
selected.
2.2.4 Experiment of concentration
0.1, 0.2, 0.4, 1, 2, 3 ml of [Rh(H2O)6]·(ClO4)3
solution containing 100 ug/mL of rhodium(III ) was added to 9.9, 9.8, 9.6, 9, 8, 7 mL of 1
mol·dm-3- NaClO4 solution respectively. The pH values of all
solutions were adjusted to 2 with perchloric acid and sodium hydroxide. The scanning rate
is 80 mv/s and the sensitivity is 100 uA/v. Using glassy carbon electrode as working
electrode, the cyclic voltammograms were recorded from +0.4 to -0.8 v (vs. Ag/AgCl).
2.2.5 Cyclic voltammetric characteristics of other species
For trans-[Rh(H2O)4Cl2]+ and cis-[Rh(H2O)4Cl2]+
species, mer-[Rh(H2O)3Cl3] and fac-[Rh(H2O)3Cl3]
species , 1 mL of their solutions containing about 300 ug/ml rhodium(III) were added to 9
ml of 1 mol·dm-3- sodium perchlorate solution respectively. With regard
to cis-[Rh(H2O)2Cl4]- species, 1 mL of
its solution containing 250 ug/mL rhodium was added to 9 mL of 0.5 mol·dm-3
ice cold KCl solution . As to [Rh(H2O)Cl5]2- and [RhCl6]3-
species, the ionic strength are 1 and 6 mol·dm-3- KCl solution
respectively. The concentrations of rhodium(III ) for them are 20 and 21 ug/mL
respectively. The pH values of all these solutions were adjusted to 2 with potassium
hydroxide and perchloric acid. The cyclic voltammograms of all species at different
scanning rate (40, 50, 60, 70, 80, 90, 100, 110, 120 mv/s respectively) were recorded from
+0.4 to –0.8 v (vs. Ag/AgCl). The sensitivity is 100
uA/v.
 |
 |
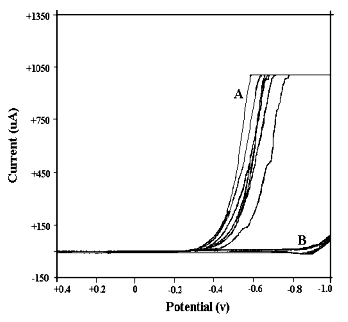
| Fig. 1 Cyclic
voltammograms of background solutions on platinum electrode A--in perchloric acid medium (0.1, 0.5, 1, 2 mol·dm-3, respectively). B--in sodium perchlorate medium (0.1, 0.5, 1, 2 mol·dm-3, respectively). |
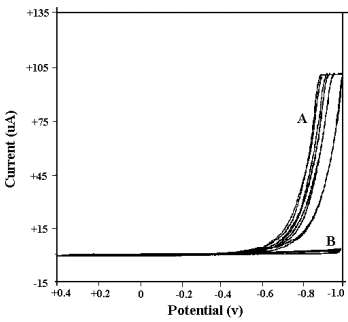
Fig. 2 Cyclic voltammograms
of background on glassy carbon electrode A--in perchloric acid medium (0.1, 0.5, 1, 2 mol·dm-3, respectively). B--in sodium perchlorate medium (0.1, 0.5, 1, 2 mol·dm-3, respectively). |
3.
RESULTS AND DISCUSSION
3.1. Study on the Cyclic Voltammetric Behavior of
[Rh(H2O)6]3+
3.1.1. Influence of different working electrode
Under the different background conditions, a series of cyclic voltammograms were recorded
by using glassy carbon electrode and platinum electrode as working electrode, respectively
(see Fig.1 and Fig. 2). Seen from these figures, the current response of background
solution on glassy carbon electrode is not in agreement with that on platinum electrode,
although their cyclic voltammograms in neutral sodium perchlorate medium is identical. In
perchloric acid medium, when scanning is performed in negative direction, the response
current sharply increases from -0.4 v to final point
potential (-1.0 v) on platinum electrode, while on glassy carbon electrode, the response
current sharply increases from -0.7 v
to final point (-1.0 v). At the same time, the shape of curve attained on glassy carbon
electrode is better than that on platinum electrode. So, in the investigation of cyclic
voltammetric behavior, it is better to choose GCE (glassy carbon electrode) as working
electrode, rather than platinum electrode.
3.1.2. pH influence
Different acidities (pH = 2, 3, 4, 5, 6, 7) were compared in sodium perchlorate medium.
For [Rh(H2O)6]·(ClO4)3 complex, no
response was obtained when pH﹥3 (see Fig.3). The
reduction peak at about -0.2 v appeared in a pH=2 solution. In fact, according to Van Loon
et al [12], the hydrolysis starts at pH greater than 2.9. So, under neutral and
alkaline conditions, the [Rh(H2O)6]3+ species has changed
into other compounds, such as hydroxide. At the same time, if the pH value is less than 2,
a great deal of bubble was observed on the surface of glassy carbon electrode. So, as a
compromise between sensitivity and selectivity, pH 2 was employed in all subsequent work.
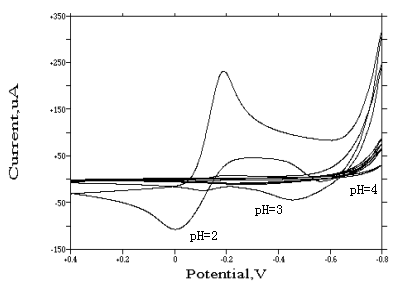
Fig. 3 Cyclic voltammograms of
[Rh(H2O)6]·(ClO4)3 at different pH
valueson glassy carbon electrode
( In 1 mol·dm-3 sodium perchlorate medium, concentration of rhodium is
10 ug/mL, scan rate is 70 mv/s, sensitivity is 100 uA/v. At pH=2, the current response is
maximum, while pH=7, the current response is minimum.)
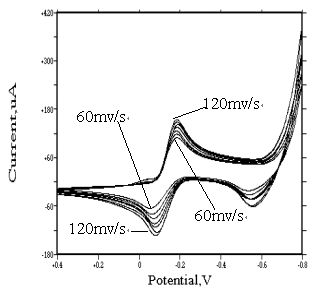
3.1.3 Study of scanning rate
Seen from Fig.4, ipa(anodic peak current)
and ipc(cathodic peak current) increase with increasing scanning rate. However,
the Epa(anodic peak potential) decreases as the scanning rate increases, while
Epc(cathodic peak potential) is still at about –185 mv under the same condition. The increase of scanning
rate provoked a decrease in the D E(Epa-Epc) value. The resulting
plot ipc vs n 1/2 is linear in the range between 60 and 120 mv/s
(see Fig.5). This behavior indicates that diffusion controlled redox process occurs in the
system. At the same time, another phenomenon has been found, that due to the reduction,
trace amount metallic rhodium on the surface of glassy carbon electrode produce weakly
adsorption.
 |
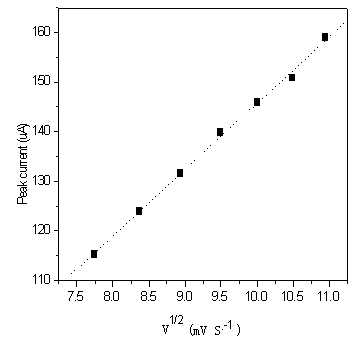 |
| Fig. 4 Cyclic voltammograms of [Rh(H2O)6]·(ClO4)3 at different scanning rate | Fig. 5 Dependence on the reduction peak current at a function of v1/2 obtained for [Rh(H2O)6]·(ClO4)3 |
| (1 mol·dm-3 NaClO4, pH=2, concentration of Rh(III) is equal to 10 ug/mL, sensitivity is 100 uA/v.) | |
3.1.4 Effects of the rhodium(III)
concentration
The reduction peak of rhodium(III) appears at different potential for different
concentration solutions of rhodium(III) (1, 2, 4, 10, 20, 30 ug/ml, respectively). When
the concentration of rhodium(III) is 1 ug/ml, the peak potential is about –0.4 v, while the concentration reaches 30 ug/ml, the peak potential
is about –0.2 v (Fig. 6). This indicates that the
reduction peak will drift with varying the concentration of rhodium(III). For the
oxidation peak, the same result is attained. So, it is difficult to determine rhodium by
utilizing the reduction (or oxidation) peak height in relationship with concentration of
rhodium(III). Similar to the phenomenon mentioned above, a weak adsorption produced by
trace amount metallic rhodium was also observed on the surface of glassy carbon electrode.
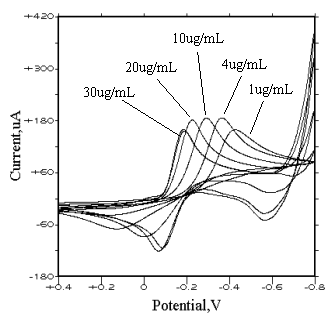
(In 1 mol·dm-3 sodium perchlorate medium, scan rate is 80 mv/s, sensitivity is 100 uA/v, pH value is 2, concentrations of rhodium(III) are 1, 2, 4, 10, 20, 30 ug/mL, respectively.)
3.2 Cyclic Voltammetric Behavior of Other Complexes
3.2.1. Cyclic Voltammetric Behavior of cis-(or trans-)[Rh(H2O)4Cl2]+
isomers
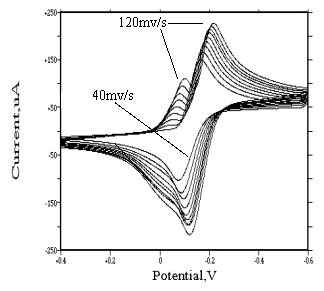
For all other chloroaquorhodium(III) complexes,
various conditions including pH 2, different scanning rate (40, 50, 60, 70, 80, 90, 100,
110, 120 mv/s, respectively) and sensitivity 100 uA/v were employed in all subsequent
work, as a better selectivity. Seen from Fig.7 and Fig.8, we can find that, for trans-[Rh(H2O)4Cl2]+
and cis-[Rh(H2O)4Cl2]+ species, at
lower scanning rate, there only exists irreversible redox process. According to the
adsorbate on the surface of glassy carbon electrode, the process may be rapid three
electrons transfer:
cis(or trans)-[Rh(H2O)4Cl2]+ +
3e « Rh + 4H2O + 2Cl-
But when the scanning rate increases, another wave appears
before the reduction wave, which gets more and more obvious with increasing
scanning rate. Refer to the theory[13,14], this is an adsorption wave
caused by the reducing of cis(or trans)-[Rh(H2O)4Cl2]+
(not being adsorbed on the surface of electrode) into metallic rhodium (being adsorbed on
the surface of electrode). At the same time, according to the strength of adsorption wave,
it can be found that the adsorption of product of cis-[Rh(H2O)4Cl2]+
on the surface of electrode is stronger than that of trans-[Rh(H2O)4Cl2]+.
 |
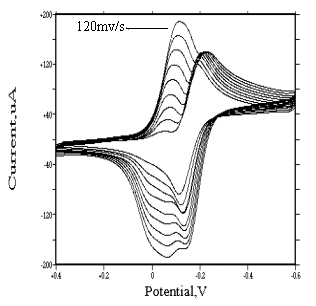 |
Fig. 7 Cyclic
voltammograms of |
Fig. 8
Cyclic voltammograms of |
(In 1 mol·dm-3 sodium perchlorate medium, the pH value of two solutions is 2, sensitivity is 100 uA/v, the working electrodes of them are both glassy carbon electrode) |
|
3.2.2 Cyclic Voltammetric
Behavior of mer-(or fac-)[Rh(H2O)3Cl3] isomers
For mer-[Rh(H2O)3Cl3] and fac-[Rh(H2O)3Cl3]
species, similar irreversible redox peaks appear at about –0.2 and -0.13 v respectively. At lower scanning rate, no adsorption
wave was found. But when scanning rate increases, an adsorption wave appears before the
reduction wave. Its strength increases as the scanning rate increases (see Fig.9 and
Fig.10). For the same reason, this adsorption wave is caused by the reducing of mer(or
fac)-[Rh(H2O)3Cl3] (not being adsorbed on the
surface of electrode) into metallic rhodium (being adsorbed on the surface of electrode).
The electrode reaction is as follows:
mer(or fac)-[Rh(H2O)3Cl3] + 3e
Comparing the strength of their adsorption wave, we can draw a conclusion that they have identical strength of adsorption.
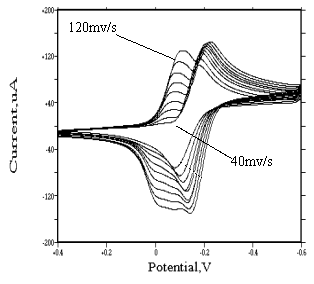 |
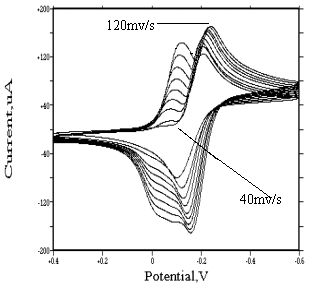 |
Fig. 9 Cyclic
voltammograms of |
Fig. 10 Cyclic
voltammograms of |
| (In 1 mol·dm-3 sodium perchlorate medium, the pH value of two solutions is 2, sensitivity is 100 uA/v, the working electrodes of them are both glassy carbon electrode) | |
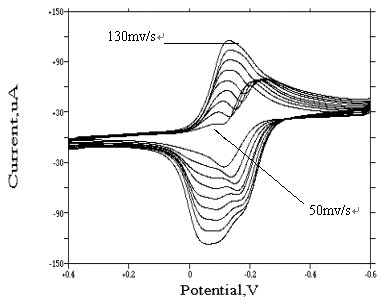
Fig. 11 Cyclic voltammograms of
cis-[Rh(H2O)2Cl4]- at different scan rate
(In 0.5 mol·dm-3 potassium chloride medium, concentration of rhodium is
25 ug/mL. sensitivity is 100 uA/v. the pH value of solutions is 2 )
3.2.3 Cyclic Voltammetric
Behavior of cis-[Rh(H2O)2Cl4]-
With regard to cis-[Rh(H2O)2Cl4]-
species, its cyclic voltammogram shows in Fig. 11 (scanning rate is 50, 60, 70, 80, 90,
100, 110, 120, 130 mv/s, respectively). Seen from Fig. 11, at lower scanning rate, similar
irreversible redox peaks appear at about –0.2 v and –0.13 v respectively. The rapid three electrons transfer caused this
result. Its electrode reaction is as follows:
cis-[Rh(H2O)2Cl4]- + 3e « Rh + 2H2O + 4Cl-
However, when the scanning rate increases to 130 mv/s, a
distinct adsorption wave appears before the reduction wave. Different from other
complexes, at the final scanning rate (i.e. 130 mv/s), the adsorption wave is so strong
that the reduction wave was covered by it. According to the theory[12], it is
obviously caused by the reducing of cis-[Rh(H2O)2Cl4]-
(not being adsorbed on the surface of electrode) into metallic rhodium (being adsorbed on
the surface of electrode strongly).
3.2.4 Cyclic Voltammetric Behavior of [Rh(H2O)Cl5]2- and
[RhCl6]3-
As to the [Rh(H2O)Cl5]2-
and [RhCl6]3- species, because of their extremely instability in
sodium perchlorate
medium, so, potassium chloride solutions 1 and 5 mol·dm-3 were adopted.
Seen from Fig. 12 and Fig.13, two series of cyclic voltammograms are identical to that of
[Rh(H2O)6]3+ (see Fig. 4). Similar irreversible redox
peaks appear at about –0.2 v and –0.05 v respectively. Their electrode reactions are as follows:
[Rh(H2O)Cl5]2- + 3e « Rh + H2O + 5Cl-
[RhCl6]3- + 3e « Rh + 6Cl-
The peak current increases with increasing the scanning
rate. Furthermore, at higher scanning rate, no adsorption wave appears. However, trace
metallic rhodium was found on the surface of glassy carbon electrode, which indicates
that, although the product is weakly adsorbed on the electrode, the diffusion-controlled
process predominates.
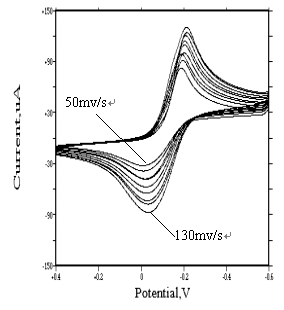 |
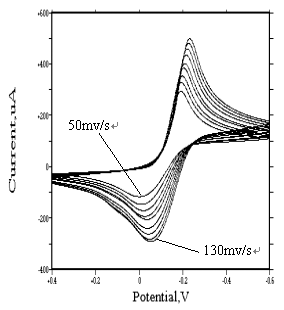 |
Fig. 12
Cyclic voltammograms of |
Fig. 13 Cyclic voltammograms of [RhCl6]3- at different scan rate (concentration of rhodium is 21 ug/mL) |
(Their media are 1 and 5 mol·dm-3 potassium chloride solutions, respectively. The pH value of two solutions was adjusted to 2. Sensitivity is 100 uA/v. The working electrodes of them are both glassy carbon electrode. Before measurement, their solutions were stored in refrigerator.) |
|
4 CONCLUSION
In this work, the cyclic voltammograms for chloroaquorhodium(III) complexes at
different scanning rate were recorded. Nearly for all complexes, rapid irreversible three
electrons transfer occurs on glassy carbon electrode. Their peak potentials appear at
about -0.2 v and -0.13
v respectively. General electrode reaction equation is as follows:
[Rh(H2O)nCl6-n]n-3 + 3e
For [Rh(H2O)6]3+ species, the affecting factors from acidity, scanning rate, concentration of rhodium solution and background were studied in detail. The optimized conditions including pH=2, glassy carbon working electrode, 70 mv/s scanning rate and 1 mol·dm-3 sodium perchlorate medium were obtained. For other complexes, we found that in the cyclic voltammograms recorded, apart from the reduction wave caused by rapid three electrons reduction, before it an adsorption wave appears, its strength of adsorption and response varies with the complex species. REFERENCES
[1] Van Loon G, Page J A. Talanta, 1965, 12: 227.
[2] Pantani F. J. Electroanal. Chem., 1963, 5: 40.
[3] Kravtsov V I, Shereshevskaya I I. Electrokhimiya, 1971, 7: 1677.
[4] Cozzi D, Pantani F. J. Electroanal. Chem., 1961, 2: 72.
[5] Pantani F. Ric. Sci., 1963, 33 (-A): 217.
[6] Hartley F R. Recent developments in the chemistry of rhodium in high oxidation state, Chemistry of the Platinum Group Metals. 1991.
[7] Beran P. Chem. Zvesti., 1960, 14: 735.
[8] Douglas W H, Magee R J. J. Electroanal. Chem., 1963, 5: 171.
[9] Gao X X. The catalytic hydrogen waves of platinum group elements, Polarographic Catalytic Waves, Science Press, 1991.
[10] Zheng J B, Liu P, Gong N et al. Fenxi Huaxue., 1998, 28(8): 1014.
[11] Gu Y W, Dong S A, Wang S X et al. Precious Metals., 2003, 24(2): 11.
[12] Van Loon J, Page J. Can. J. Chem., 1961, 44: 515.
[13] Wopschall R H, Shain I. Anal. Chem., 1967, 39(13): 1514.
[14] Anson F. Electrochemistry and Electroanalytical Chemistry. Translated by Huang Wei et al, Peking University Press, 1981.
铑(III)氯水配合物的循环伏安行为研究
顾永万a, 董守安b, 张慧颖c, 段德良d, 王士兴b
(a昆明贵研催化剂有限责任公司,650101; b昆明贵金属研究所,650221; c昆明理工大学地质系,650093; d北京大学化学系,100871)
摘要 通过对不同种态和不同浓度的铑(III)氯水配合物在不同的pH值、电压范围、扫描速率下进行循环伏安行为研究,结果表明,以玻炭电极为工作电极,它的最佳条件为:pH=2;扫描速率为70毫伏/秒;[Rh3+]=20微克/毫升;1mol/L的高氯酸钠介质体系。同时发现,对几乎所有的种态,在玻碳电极上都会发生快速的三电子转移,电极反应为:[Rh(H2O)nCl6-n]n-3 + 3e « Rh + nH2O + (6-n)Cl- 它们的峰电位分别约为-0.2v和-0.13v,但是,峰高并不与[Rh3+]成正比,因此不能用于铑的测定。此外,除了[Rh(H2O)Cl5]2- 和[RhCl6]3+种态外,其它的种态均出现两个峰。根据吸附理论,我们认为前一个峰为不吸附的反应物还原成被强吸附的产物所致的。
关键词 铑(III)氯水配合物; 循环伏安法; 电分析