http://www.chemistrymag.org/cji/2005/071006ne.htm |
Jan.21, 2005 Vol.7 No.1 P.6 Copyright |
Peroxidase activity, immobilization and application of hemalbumin
Li Renqiang, Zhang Xiaofen, Ma Aineng, Xu Siguang
(Department of Biotechnology, Jinan University, Guangzhou, 510632, China)
Received Nov. 9, 2004; Supported by the Grant-in-Aid for scientific research to Li R from Jinan University, China (640073).
Abstract Equimolar bovine serum albumin
and hemin were combined in vitro to form hemalbumin, of which the peroxidase activity was
examined and compared to that of those heme-containing compounds. Results showed that the
peroxidase property of hemalbumin was almost the same as those native heme-containing
proteins in vivo such as hemoglobin and myoglobin, and hemalbumin was a stable, active
molecule. Then hemalbumin was immobilized to the activated vehicle Sepharose 4B with the
couple ratio of 5.3mg hemalbumin per gram vehicle to make hemalbumin column, which was
applied to measure trace hydrogen peroxide. Hemalbumin column presented a high measurement
sensitivity, a stable peroxidase property with normal activity that may assay the lower
concentration of hydrogen peroxide as low as 3.63×10-7 mol/L. This study on
synthesized hemalbumin was valuable in clinic and environmental chemistry.
Keywords Hemalbumin, Peroxidase activity, Immobilization, Sepharose 4B, Hydrogen
peroxide
As an abundant protein in life body, albumin
functions as an important substance that transport an incredible variety of endogenous and
exogenous compounds, including hemin released from hemoglobin [1]. These
properties of albumin are due to that it is an active molecule composed of a polypeptide
with abundant disulfide bonds and active amino acids. So it is easy in vitro to combine
hemin with albumin to form hemalbumin, of which many studies have been performed [2-6].
Although the hemin activity is decreased when it is combined into albumin [5],
hemalbumin possesses peroxidase activity due to the hemin in the molecule [6].
However, comparing those native heme-containing compounds, such as hemoglobin (Hb),
myoglobin (Mb), cytochrome c (Cyt c), peroxidase (POD), which present also the peroxidase
activity, whether the peroxidase activity of hemalbumin that is synthesized in vitro is
different? To probe this difference would be helpful to understand furthermore the
character of hemalbumin.
On the other hand, making this enzymatic property of hemalbumin in
application is significant because of its cheapness in synthesis as compared with the
obtaining for those biological enzymes. So the immobilization of the hemalbumin was a good
idea due to that enzymatic immobilization has many advantages as known. Considering that
albumin is an active protein, and a vehicle Sepharose 4B that has higher affinity to
protein after it is activated, the study on the combination between hemalbumin and
Sepharose 4B to immobilize hemalbumin deserves to be performed.
This paper describes the comparison among those native and synthesized
in vitro heme-containing compounds in peroxidase activities, and the process to combine
hemalbumin with Sepharose 4B to make a mimic peroxidase column. The couple ratio for the
immobilization of mimic peroxidase and its application for the trace hydrogen peroxide
measurement are presented too.
1 EXPERIMENTAL
1.1 Materials and instruments
Hemoglobin human, myoglobin from horse skeletal muscle, cytochrome c from horse heart,
horseradish peroxidase and hemin chloride were from Sigma. Bovine serum albumin (BSA),
Sepharose 4B, o-phenylemediamine, 30% hydrogen peroxide and sodium dithionite were from
Biochemical Company BOAO (Shanghai, China) or from Guangzhou Chemical Company (Guangzhou,
China). All reagents were reagent grade and used as received. Solvent was distilled water.
The instrument for absorption spectral scan was UV-VIS Spectrumlab 54 (Shanghai Lingguang,
China).
1.2 Combination of hemin and BSA
Hemin chloride was first dissolved in some 15% ammonia water then in distilled water
to make 2.0% mother solution. Hemin chloride solution was dropped slowly into the BSA
solution stirred at 30oC with 0.60mM/L
equimolar as their final concentrations (our preliminary test convinced that hemin may be
combined completely by BSA in equimolar), kept at 30oC for 30 min. The visible absorption spectra of the mixture were
examined for convincing the combination and its pH was adjusted to 9.5 using 0.5N HCl or
0.05mol/L NaOH.
1.3 Measurements of peroxidase activities for those heme-containing compounds
Hydrogen peroxide may oxidize o-phenylenediamine to form 2,3-diaminophenazine that has
an absorption peak at 428nm [7, 8], which will be accelerated greatly by
peroxidase that has violent activation function to hydrogen peroxide. This reaction will
also be speeded by heme-containing proteins accounted for the function of heme. So the
reaction rate of this process may refer to the peroxidase activities of different
heme-containing compounds. Excessive substrates o-phenylenediamine, hydrogen peroxide were
used for the examination of peroxidase activity. The heme-containing compound samples were
in the same heme concentration 0.30mmol/L, but horseradish peroxidase was great diluted
due to its great enzymatic activity. After 2ml 20mmol/L o-phenylenediamine was mixed with
the sample then with 1ml 20% hydrogen peroxide, immediately the O.D.428 was
read in 1 min using time scan, a cell path length 1 cm at room temperature. Each sample
adopted was 5, 10, 15, 20, 25, 30 and 35 ml, respectively. O.D.428 were examined several times for
each sample and recorded with an average of 4 times. Obviously, O.D.428 at 1
min reflects the activation abilities to hydrogen peroxide of various heme-containing
samples with different concentrations.
1.4 Immobilization of hemalbumin on Sepharose 4B
Sepharose 4B was first activated before combination with hemalbumin. 8g of Sepharose
4B was washed using 100ml 1mol/L NaCl and 100ml water, respectively, then mixed with 6.5ml
2mol/L NaCl, 1.5ml epichlorohydrin and 15ml 56% 1, 4-dioxane. Mixture was shaken at 40oC
for 2 hours activation, washed with water and the solution of
0.2mol/L, Na2CO3-NaHCO3 buffer with pH9.5, respectively.
Some synthesized hemalbumin solution (known concentration) was mixed with the Sepharose
4B, shaken at 40oC for about 24 hours then
filtered. The hemalbumin in eluent that was not combined to the vehicle was measured using
heme absorption spectrum to calculate the couple ratio. Mixture of hemalbumin with
Sepharose 4B was put in the column after it was saturated in 0.2mol/L of pH9.5 Na2CO3-NaHCO3
buffer. Replacing activated Sepharose 4B by inactivated one as control.
1.5 Application of immobilized hemalbumin for measurement of trace hydrogen peroxide
Based on that heme-containing compounds may catalyze hydrogen peroxide stated above,
trace hydrogen peroxide was mixed with 3ml 20mmol/L o-phenylenediamine and passed the
hemalbumin column controlled by peristaltic pump with flow rate 0.5ml/min (eluant was
0.2mol/L, pH9.5 Na2CO3-NaHCO3 buffer). O. D.428
of eluent was examined. Combining water with o-phenylenediamine and performed at the same
action as the control. These experiments were also done with different column pH values
adjusted using Na2CO3-NaHCO3 buffer in pH 9.1-10.1.
2. RESULTS
2.1 Comparison of peroxidase activity of hemalbumin to native heme-containing compounds
Fig.1 showed the absorption spectrum of mixture of albumin with hemin in reduced form
reduced by small quantities of sodium dithionite, which indicated that the hemin had been
combined by albumin to form hemalbumin.
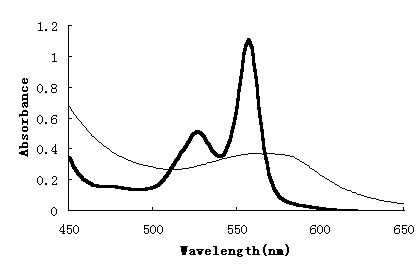
Fig.1 Absorption spectra of mixture (albumin with
hemin, bold solid line) and hemin only (thin solid line) in ferrous reduced state in
450-650 nm region. Both samples were in equimolar 0.15 mmol/L hemin concentration and at
same pH9.5. Light bath length was 0.5 cm.
Fig, 2 showed the
activation abilities of those heme-containing compounds to hydrogen peroxide. Peroxidase
was of course the highest as several hundred folds as that of the other heme-containing
compounds in activation ability. This indicated its different mechanism from the other
heme-containing proteins in catalysis reaction. For those heme-containing proteins,
Cytochrome c presented the highest peroxidase activity and myoglobin the lowest. Catalysis
ability of hemalbumin had little difference from hemoglobin and myoglobin, implying that
they had the same mechanism in activation reaction and hemalbumin was a stable, active
molecule. These results indicated that it was significant for further study in
immobilization of this synthesized mimic peroxidase.
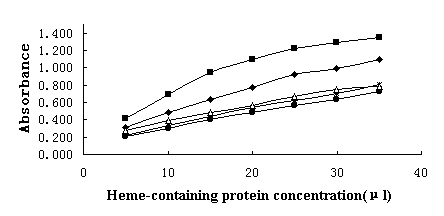
Fig. 2 O.D.428 of catalytic reaction read
at 1 min for heme-containing compounds.
From top to bottom: Cyt c, POD, hemalbumin, Hb and Mb.
Substrates 20 mM/L o- phenylemediamine 2 mL, 20% H2O2
1 mL, measured at room temperature using 1 cm light bath length. All samples were in
equimolar 0.3 mmol/L heme concentration except POD that was only 0.0012 mmol/L heme
concentration.
2.2 Immobilization of hemalbumin
Hemalbumin was immobilized well on Sepharose 4B vehicle after the activation of
Sepharose 4B, the activated vehicle may combine greatly hemalbumin molecule comparing to
inactivated one, of which the couple ratio was obtained by calculation as the following:
Couple ratio between hemalbumin and inactivated Sepharose 4B:
0.529/0.745×0.0978mg/ml×13ml = 0.902mg
(0.978mg―0.902mg) /8g = 0.010mg
heme/g Sepharose 4B
Couple ratio between hemalbumin and activated Sepharose 4B:
0.273/0.745×0.0978mg/ml×16ml = 0.573mg
(0.978mg―0.573mg) /8g = 0.051mg
heme/g Sepharose 4B
Here the couple ratio was presented as heme quantity/per gram vehicle,
if changed to hemalbumin, the couple ratio for activated Sepharose 4B was 5.3mg hemalbumin
per gram vehicle. This couple ratio was quite high in our knowledge.
2.3 Measurement of trace hydrogen peroxide by hemalbumin column
After the hemalbumin column (0.5×8cm) was equilibrated with 0.2mol/L, pH9.5 Na2CO3-NaHCO3
buffer, it was applied to measure trace hydrogen peroxide. As the concentration of
hydrogen peroxide in the sample increased according to linear proportion in 3.63×10-5―3.63×10-4mol/L, O.D.428 of eluent was in
high correlativity to the concentration of hydrogen peroxide with r = 0.9933, linear
equation F = 0.0092+0.0012[H2O2]. Relative standard deviation (RSD)
was 2.2% when the examination was done for 10 times using 9.083×10-5mol/L
hydrogen peroxide. These results indicated that hemalbumin was a stable, active molecule
after it was immobilized on Sepharose 4B vehicle and presented the mimic peroxidase
property. This hemalbumin column may assay the lower concentration of hydrogen peroxide as
to 1.08×10-8mol/L according to the calculation by the equation but in actual
practice, 3.63×10-7mol/L hydrogen peroxide was clearly checked out. Changing
the column pH value using the buffer from 9.1 to 10.1, the sensitivity for measurement of
trace hydrogen peroxide changed a little.
3 DISCUSSION
Hemalbumin is a synthesized in vitro organic molecule. The peroxidase property of this
synthesized molecule due to the hemin in the molecule is almost the same as those native
heme-containing proteins in life body demonstrated by the present study. So the
application of this synthesized molecule is significant due to lower cost to obtain this
substance, which makes the immobilization of hemalbumin as a main aim in the study. The
immobilization of hemalbumin to Sepharose 4B is successful from the couple ratio and its
action properties in application of measurement to trace hydrogen peroxide. During the
measurement of hydrogen peroxide using the column, the sensitivity decreased after several
times of examinations or one time in high concentration of hydrogen peroxide, and the
color of column changed. If the column was treated with sodium dithionite solution, the
sensitivity of measurement and the color were recovered fully but if treated with
potassium ferricyanide, not any recovery happened. These indicated that valence change of
hemin iron during activation to hydrogen peroxide was from +3 to +5 but not from +3 to +2
as presented in normal redox reaction catalyzed by hemin iron. Sodium dithionite reduced
the iron from +5 back to +3. This process may be the mechanism for those heme-containing
proteins in catalysis to hydrogen peroxide.
The chemical character of hemalbumin column was stable, which was
presented by the column in keeping high sensitivity of measurement to trace hydrogen
peroxide even if the column existed at room temperature for long time. The measurement
sensitivity of the column was mainly related to column length, the longer column has
higher sensitivity than that of shorter length column but this relationship was not a good
linear one, which may imply the complex of the reaction happened between hydrogen peroxide
and the column. In different lengths of longer column, the sensitivity showed no
difference.
Manufacture of the hemalbumin column makes the measurement of trace
hydrogen peroxide for biological and inorganic samples possible in quick and large numbers
of samples, which would be useful for clinic and environmental chemistry. This hemalbumin
column would have some other applications due to its stable peroxidase property and the
valence change of hemin iron that may change from +3 to +5 or to +2, for example, for the
measurement of antioxygenic activity to some biological substances, which is obviously
good for the study on healthful food and medicine that is also the work we will continue.
[1] Peters JR T. All about Albumin, California: Academic Press, Inc., 1996: 234-240.
[2] Marco M, Alessandro V, Massimo C et al. J. Inorg. Biochem. 2001, 84: 293-296.
[3] Mauro F, Marco M, Massimo C et al. J. Inorg. Biochem. 2002, 91: 487-490.
[4] Wu Y, Teruyuki K, Eishun T. Inorg. Chimi. Acta. 2001, 322: 120-124.
[5] Grinberg L N, O'Brien P J, Hrkal Z. Free Radic. Biol. Med. 1999, 26 (1/2): 214-219.
[6] Monzani E, Bonafe B, Fallarini A et al. Biochim. Biophys. Acta. 2001, 1547: 302-312.
[7] Peter J T, Victor P C, Dave W. Anal. Biochem. 1987, 165: 230-233.
[8] Jiao K, Sun G, Zhang S S. Science in China (Series B) 1998, 41: 345-352.
血红白蛋白的过氧化物酶活性、固定及其应用
李任强,张小芬,马霭能,徐思光
(暨南大学生物工程学系,广州 510632)
摘要 在体外将牛血清白蛋白与氯化血红素以相同摩尔量结合制成血红白蛋白,测定了其过氧化物酶活性,并与天然的含血红素的物质的活性进行了比较。结果表明血红白蛋白与生物体内的血红蛋白、肌红蛋白具有几乎一样的过氧化物酶特性,是一稳定而又活泼的分子。将血红白蛋白固定在已活化的Sepharose 4B载体上制成血红白蛋白柱子,偶联率达每克载体结合5.3mg血红白蛋白。将血红白蛋白柱子应用于测定微量过氧化氢,显示正常、稳定的过氧化物酶活性,具极高测定灵敏度,能明显的测出低至3.63×10-7mol/L浓度的过氧化氢。本研究对临床化学和环境化学都具有价值。
关键词 血红白蛋白,过氧化物酶活性,固定化,Sepharose 4B,过氧化氢