http://www.chemistrymag.org/cji/2005/072014ne.htm |
Feb.1, 2005 Vol.7 No.2 P.14 Copyright |
Yang Kewu, Jiang Xuanzhen
( Department of Chemistry, University of Zhejiang, Hangzhou, 310027, China )
The project was supported by the National Science Foundation of China (No: 20376071)
Abstract The asymmetric hydroesterification of
styrene into 2-phenyl propionate by homogeneous catalytic system of PdCl2, CuCl2,
oxygen and HCl was studied under very mild conditions ( room temperature and atmospheric
pressure ). Quite high conversion of styrene (97%), relatively low regioselectivity ( e.g.
b/n, 70/30) and modest asymmetric inductions (15-38% ee) were obtained by using (S)-(+)-1,1'-binaphthyl-2,2'-diyl
hydrogen phosphate as the chiral ligand. The presence of CuCl2 and O2
as final reoxidant of palladium is necessary.
Keywords asymmetric
hydroesterification, chiral BNPPA ligand, styrene
The hydroesterification of styrenoids by using carbon monoxide and alcohols produces industrially valuable intermediates for perfumes and pharmaceuticals. In the past decases excellent progresses on asymmetric hydrogenation has been made . However the progress on the asymmetric hydroesterification of styrenoids is still limited so far. Hayashi's pioneering work[1] using Pd(dba)2-neomenthyldiphenylphosphine-trifluoroacetic acid in the asymmetric hydroesterification of methylstyrene showed moderate ee ( 42 % ee; b/n = 94:6; dba = dibenzylideneacetone) . Recently Inoue et al. [2] used a chiral ferrocene, containing aminophosphine, (S)-(R)-BPPFA , ((S)-1-[(R)-1',2-bis(diphenylphosphino)-ferrocenyl] ethyldimethylamine) in the asymmetric hydroesterification of styrene and achieved a high asymmetric induction of 86 % ee, but rather low yield and regioselectivity. Very recently Wang et al. [3 ] reported the palladium-catalyzed asymmetric hydroesterification of styrene by using the planar-chiral ferrocene oxazoline ligands. Excellent regioselectivity (b /n > 99:1) with low ee (28 %) was achieved in the presence of CuCl2; while in the case of absence of CuCl2 moderate enantioselectivity ( 64% ee ) but low regioselectivity ( b/n = 40/60) was obtained. However on these investigations the stringent condition, for instance 1800 psi CO pressure was required.
In the present study (S)-(+)-1,1'-binaphthyl-2,2'-diyl hydrogen phosphate, ((S)-(+)-BNPPA), was employed in the palladium-catalyzed asymmetric hydroesterification of styrene at room temperature and atmospheric pressure. Under the same reaction conditions, the same chiral ligand was used for palladium-catalyzed asymmetric hydrocarboxylation of olefins by Alper et al. [4 ] rather than for the asymmetric hydroesterification of olefins. Therefore in this study the hydroesterification of styrene catalyzed by PdCl2-CuCl2 with (S)-(+)-BNPPA, under very mild conditions has been firstly developed, although the relatively low regioselectivity (for instance b/n, 70/30) and modest asymmetric inductions (15- 38 % ee) were obtained.
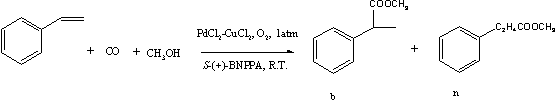
Scheme 1 2. RESULTS AND DISCUSSION
The asymmetric hydroesterification reaction of styrene is shown in Scheme 1. Enantiomeric branched ester (b) is the desired product while the ester (n) is considered to be a byproduct. The experiments were carried out under similar reaction conditions previously described by Alper et al. [4] by bubbling carbon monoxide and oxygen with certain flow rates, into a solution of PdCl2, CuCl2, (S)-(+)-BNPPA, HCl (liquid or anhydrous) and styrene in the solvent at room temperature and atmospheric pressure. The experimental results at different reaction conditions are summarized in Table 1.
Table 1 Asymmetric hydroesterification of styrene using PdCl2-CuCl2-(S)-(+)-BNPPA catalyst system(a)
Entry |
solvent |
HCl |
Cu/Pd(M/M) |
Conv.(%)(b) |
b/n(b) |
(S)ee%(b) |
1 |
CH3OH |
anhydrous |
4/1 |
29 |
77/23 |
15 |
2 |
CH3OH |
anhydrous |
6/1 |
54 |
78/22 |
16 |
3 |
CH3OH |
anhydrous |
8/1 |
97 |
70/30 |
38 |
4 |
THF |
anhydrous |
8/1 |
55 |
91/9 |
23 |
5 |
1,4-dioxane |
anhydrous |
8/1 |
2 |
64/36 |
- |
6 |
CH3OH |
35% |
8/1 |
62 |
54/36 |
29 |
7 |
THF |
35% |
8/1 |
19 |
80/20 |
18 |
8(c) |
CH3OH |
anhydrous |
8/1 |
75 |
66/34 |
16 |
(b) The data on conversion and regioselectivity were determined by GC with capillary column O.V.-101(30 m). The enantiomeric excess of the chiral product was determined by HPLC (Waters-2690) with a chiral column (S, S-whelk-01). The absolute configuration of methyl 2-phenylpropionate was determined by the comparison of the retention time with that of a pure authentic sample.
(c) Doubling the amount of (S)-(+)-BNPPA to 0.76 mmol
As CuCl2
was found as the most efficient reoxidant, the influence of its concentrations was checked
as shown in the entries 1-3 of Table 1. One can see therein that the increase in the ratio
of Cu/Pd (from 4/1 to 8/1) gave higher conversion of styrene (97 %), asymmetric induction
(38 % ee) and regioselectivity ( b/n, 70/30) . Whereas an excess of CuCl2 will
decrease the efficiency of the reaction as reported in the reference[5].
Influence of the solvents on the performance of reaction was presented in the entries 3-5,
indicating that methanol performed quite well compared with others ( THF and 1,4-dioxane).
Hydrochloric acid is necessary for the palladium catalyzed asymmetric
hydroesterification of styrene, however its role is not yet well understood. From the
comparison of entry 3 with 6 (methanol as the solvent) and entry 4 with 7 (THF as the
solvent) it can be seen that anhydrous HCl gave better conversion, regioselectivity and
enantioselectivity than its aqueous form. This finding has not been reported for the
asymmetric hydroesterification of styrene so far.
To test the influence of the amount of (S)-(+)-BNPPA on the
asymmetric induction on the performance of reaction, the amount of (S)-(+)-BNPPA
was doubled as shown in entry 8 of Table 1. By comparison entry 8 with 3 it can be seen
that the increase in the amount of the chiral ligand did not enhance the efficiency of the
reaction. In addition, the reproducibility of experimental results was fairly well, for
instance, twice runs of entry 3 gave optical yields of 38.1 % ee, 38.4 % ee , respectively
.
In this catalytic system the role of oxygen is to avoid the catalyst
decay under CO-O2 atmosphere. The influence of O2/CO molar ratio has
been examined. A suitable flow rates ( O2: 1.5 mL/min.; CO: 12 mL/min.) were
chosen in this study. At room temperature and atmospheric pressure the asymmetric
hydroesterification of styrene catalyzed by PdCl2-CuCl2-(S)-(+)-BNPPA
has been firstly achieved, although the relatively low regioselectivity and modest
asymmetric induction were obtained. The various performances with the aim to increase in
the regioselectivity and enanoselectivity have been made but were not successful. This may
be the reason why Alper's mild catalyst system[4] has not been applied to the
asymmetric hydroesterification reaction but to asymmetric hydrocarboxylation of olefins to
obtain the acids.
In this study it has been demonstrated that palladium-catalyzed asymmetric hydroesterification of styrene by using (S)-(+)-BNPPA as the chiral ligand at room temperature and atmospheric pressure gave fairly high conversion, relatively low regioselectivity and modest asymmetric induction . 4. EXPERIMENAL
4.1 Materials
Palladium chloride, cupric chloride and 35% HCl were purchased from Shanghai Chemical Reagent Company. Styrene, methanol and tetrahydrofuran were treated by molecular sieve (4A) before use. Anhydrous HCl was prepared by adding H2SO4 into NaCl and then gaseous hydrochloride was adsorbed via methanol. Carbon monoxide and oxygen were dried by molecular sieve cylinders before bubbling into the solution.
The chiral ligand , (S)-(+)-BNPPA as shown in Scheme 2 , was synthesized according to the literature method [6].
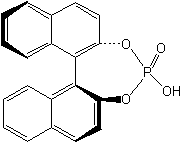
Scheme 2 (S)-(+)-BNPPA
4.2 General procedure
In a typical atmospheric pressure test, carbon monoxide (12 mL/min.) was bubbled
through a solution containing CH3OH (50 mL) and PdCl2 (1 mmol). 12
mmol (anhydrous or 35% aqueous) HCl was added, the mixture was stirred for 5 min. CuCl2
(8 mmol) was added, and then oxygen (1.5 mL/min.) was bubbled through the solution
(together with CO). After stirring for 10 min, it was followed with the addition of the
chiral ligand. Ten min. later, styrene (2.5 mL) was added. The reaction mixture was
stirred for 18 h at room temperature.
The conversion of styrene and regioselectivity (b/n) were determined by
GC with capillary column O.V.-101(30 m length). The enantiomeric excess of the chiral
product was determined by HPLC (Waters-2690) with a chiral column (S, S-whelk-01).
The absolute configuration of 2- phenylpropionate was determined by the comparison of the
retention time with that of a pure authentic sample.
REFRENCES
[1] Hayashi T, Tanaka M,
Ogata I. Tetrahedron Lett., 1978, 41: 3925.
[2] Oi S, Nomura M, AiKo T, Inoue Y. J. Mol. Catal. A: Chem. 1997, 115: 289.
[3] Wang L L, Kwok W H, Chan A S C, Tu T, Hou X L, Dai L X. Tetrahedron
Asymmetry, 2003, 14: 2291.
[4] Alper H, Hamel N. J. Am. Chem. Soc., 1990, 112: 2803.
[5] Bertoux F, Castanet Y, Civade E, Monflier E, Mortreux A, Catal. Lett., 1998, 54: 199.
[6] Jacques J, Fouquey C. Tetrahedron Lett. , 1971, 48: 4617.
杨可武 姜玄珍
(浙江大学化学系 杭州 310027)
摘要 本文对温和条件(室温、常压)下,在PdCl2/CuCl2 、O2、HCl均相催化体系中苯乙烯的不对称氢酯化反应进行了研究。以(S)-(+)-联萘酚磷酸氢酯为手性配体,得到了较高的转化率(97%)、相对较低的区域选择性(如:b/n为70/30)及一定的对映选择性(15-38% ee)。CuCl2和O2在这个催化体系中是必须的,它们对钯的氧化再生起一定作用。
关键词 不对称氢酯化 手性配体BNPPA 苯乙烯