http://www.chemistrymag.org/cji/2005/071013pe.htm |
Jan.21, 2005 Vol.7 No.1 P.13 Copyright |
(College of Chemistry and Environmental Science, Hebei University, Baoding 071002, China)
Received Sep.
26, 2004; Hebei Science and Technology Department, and Science and Technology Bureau, of Baoding city. (Project: 03547023D, 03N006)Abstract A new analytical method has
been developed to determine organophosphorus, organochlorine, carbamate and pyrethroid,
multicomponent pesticide residues in corn. It is based on a fast extraction of pesticides
with dichloromethane and a further clean-up procedure by solid-phase extraction using a
Florisil cartridge, then analysed by gas chromatography-mass spectrometry (GC-MS). The pesticides were identified by retention time and the
proportion of qualitative ions, and quantified being base on extract of spiking standards
in blank sample. The determination results of accuracy, precision and the limits of
detection (LOD) was shown that the method was validated.
Keywords GC-MS, pesticides, multi-residues, corn
1. INTRODUCTION
Corn is the main product of Chinese agriculture, and is one of the important goods in
international trade. But in the past many years, the problem that abuse of pesticides was
prevalent in China, the residue of pesticides in farm produce was becoming a neck in
export.
Two methods about
determination of pesticide residues in corn have been found. One is that determination of
organochlorine and organophosphorus pesticide residues in corn by GC with Hall
electrolytic conductivity and flame photometric detector, respectively[1]. The
other one is only determination of 11 organochlorine pesticide residues in corn by GC-MS[2].
But simultaneous determination of organophosphorus, organochlorine, carbamate and
pyrethroid pesticide residues in corn has not been reported, and the analytical methods of
pesticide residues for other cereals have a fewer reported, too, moreover these methods
were used to determine one class of pesticides. For instance, GC-FPD[3,4] or
GC-NPD[5] was used to determine organophosphorus pesticides in rice, GC-ECD[6] was
used to determine organochlorine pesticides in wheat grain, HPLC[7] was used to
determine N-Methyl carbamate pesticides in rice, and so on, or only one pesticide was determined by HPLC[8,9]
or GC-MS[10] in cereals. Nowadays, the analysis of a number of pesticides to
corn samples can guarantee the fulfillment of maximum residue levels (MRLs) legislations. The
analytical methodology required should be fast, simple and robust in order to be applied
after the appropriate validation following quality criteria in routine laboratories.
The purpose of this
article is to establish a simple, rapid and sensitive method to simultaneously determine
multi component pesticide residues in corn. Firstly, in process of extraction, the
solvents using in extraction of pesticides have been selected. Secondly, solid-phase
extraction (SPE) was used to clean-up for removing impurities. SPE was a simple, rapid
clean-up method, and was widely used in analysis of pesticide residue. The SPE sorbent in
common use included: C18, LC- aluminum-N, florisil, etc. In analysis of
multicomponent pesticide residues, the florisil cartridge was more used in process of
clean-up [11-15]. Because the polarities of these pesticides were different,
the mixture solvent of hexane and acetone was selected as the eluent. The conditions of
clean-up were confirmed by the results of experiment. Finally, the prepared sample was
determined using GC-MS in selected-ion monitoring (SIM) mode.
2. EXPERIMENTAL
2.1 Reagents and chemicals
LC grade dichloromethane, hexane, acetone were purchased from Merck (Darmstadt,
Germany), analytical grade sodium chloride and anhydrous sodium sulphate (prepared 3 h at
650℃ before using) were purchased from Beijing
chemical plant (Beijing, China). For SPE, Florisil 1g cartridges were supplied by Beijing
Zhenxiang Corporation (Beijing, China).
2.2 Apparatus
6890/5973N gas chromatography-mass spectrometry was obtained from Agilent Technologies (USA), N-EVAPTM112 Nitrogen evaporation was obtained from Organomation Associates. Inc (USA), Vacuum manifold processing station was obtained from Supelco (USA), FW-80 Disintegrator was purchased from Tianjin taisite instrument corporation (Tianjing, China), WH-861 Vortex shaker was purchased from Taicang kejiao instrument plant (Taicang, China), KQ-250B Ultrasonic bath was purchased from Kunshan Ultrasonic Instrument corporation (Kunshan, China).
2.2.1 Gas chromatography parameters
A fused-silica capillary column HP-5 MS (Agilent, USA, 30m×0.25mm I.D., 0.25m m film thickness) was used with helium as the carrier gas. Constantly pressure model, the retention time locked by methyl chlorpyrifos. A 1-m L volume of sample was injected by the autosampler applied splitless injection technique with the split closed for 0.75 min. The chromatographic temperature conditions was as follows: 70oC for 2 min, increased at 25oC/min to 150oC, 3oC/min to 200oC, then 8oC/min to 280oC final temperature, held for 10 min. The injector temperature was maintained at 220oC.
2.2.2 Mass spectrometric parameters
Mass spectrometric ion source conditions were as follows: Ion source temperature: 230oC, interface temperature: 280oC, electron voltage: 70eV. Selected-ion monitoring (SIM) was performed.
2.3 Procedures of experiment
2.3.1 Sample preparation
Crush the samples until all the fragment could through the boult with 20 mesh. The spiked samples were obtained by adding standard solution to 2.0g blank sample in which pesticide residues were not detected.
2.3.2 Extraction of sample
2.0 g sample after being milled was placed into a 50 mL beaker, 2.0 mL solution of saturated sodium chloride was added, followed by adding 10.0 mL dichloromethane, extracted by ultrasonic for 10 min. After that, 3.0 g anhydrous sodium sulfate was added and stayed for 2 min. The extraction was transferred to a column packed with 4.0 g anhydrous sodium sulfate, and rinsed twice (total 5 mL) with dichloromethane, all the eluents were collected, and evaporated to nearly dryness under nitrogen stream at 45℃, the residue was redissolved with about 1 mL hexane.
2.3.3 Clean-up procedure
A florisil SPE column was conditioned with 6.0 mL mixture of hexane-acetone (4:1) and 5.0 mL hexane, the concentrated extraction was loaded on the top of the cartridge, and followed by eluting with 6.0 mL mixture of hexane-acetone (4:1), the eluent was collected, and evaporated to nearly dryness under nitrogen stream at 45℃. The residue was redissolved in 0.5 mL hexane, and determined using GC-MS.
3. RESULTS AND DISCUSSION
3.1 Identification of the pesticides
All pesticides were identified by retention time and the proportion of selection ions (see
Table 1). Total ion chromatogram (TIC) of the mixture solution of pesticide standards was
shown in Fig. 1.
Table 1 Retention time and selection ions
Compound |
Retention time(min) |
TIon (m/z) |
Q1(m/z) |
Q2(m/z) |
Q3(m/z) |
dichlorvos |
5.83 |
109 |
185 |
79 |
187 |
isoprocarb |
9.09 |
121 |
136 |
91 |
77 |
phorate |
11.96 |
75 |
121 |
260 |
97 |
α-BHC |
12.08 |
181 |
219 |
183 |
217 |
dimethoate |
12.68 |
87 |
93 |
125 |
143 |
carbofuran |
13.03 |
164 |
149 |
131 |
123 |
β-BHC |
13.20 |
219 |
181 |
183 |
217 |
lindane |
13.46 |
181 |
183 |
219 |
111 |
pentachloronitrobenzene |
13.68 |
237 |
249 |
295 |
214 |
diazinon |
14.47 |
179 |
137 |
152 |
199 |
δ-BHC |
14.54 |
181 |
219 |
183 |
217 |
chlorothalonil |
14.78 |
266 |
264 |
268 |
270 |
methyl parathion |
16.59 |
263 |
109 |
125 |
79 |
vinclozolin |
16.63 |
212 |
285 |
198 |
187 |
carbaryl |
16.81 |
144 |
115 |
116 |
145 |
paraoxon |
17.34 |
109 |
149 |
139 |
275 |
fenitrothion |
18.07 |
277 |
125 |
109 |
260 |
pirimiphos-methyl |
18.31 |
290 |
276 |
305 |
233 |
fenthion |
19.12 |
278 |
125 |
109 |
169 |
parathion |
19.27 |
291 |
109 |
97 |
139 |
triadimefon |
19.39 |
208 |
85 |
210 |
—— |
isofenphos |
21.62 |
213 |
58 |
121 |
255 |
quinalphos |
21.62 |
146 |
157 |
156 |
118 |
op`-DDE |
22.50 |
246 |
318 |
316 |
248 |
endosulfan |
22.64 |
241 |
195 |
239 |
237 |
op`-DDD |
24.35 |
235 |
237 |
165 |
236 |
pp`-DDD |
25.69 |
235 |
237 |
165 |
236 |
ethion |
26.00 |
231 |
153 |
97 |
125 |
phosmet |
28.50 |
160 |
161 |
77 |
93 |
fenpropathrin |
28.99 |
97 |
181 |
125 |
265 |
phosalone |
29.68 |
182 |
121 |
184 |
367 |
permethrin I |
31.37 |
183 |
163 |
165 |
184 |
permethrin II |
31.55 |
183 |
163 |
165 |
184 |
fenvalerate I |
34.27 |
167 |
125 |
181 |
152 |
fenvalerate II |
34.68 |
125 |
167 |
181 |
169 |
deltamethrin |
36.00 |
181 |
253 |
251 |
255 |
3.2 Selection of the extraction solvents
2.0 g sodium chloride was placed into a 50 mL beaker. The mixture of pesticide standards
solution was added and 2.0 mL distill water was added, then 10 mL solvent was added, the
followed processing was the same as the described conditions above extraction procedure.
The residue was redissolved in 0.5 mL hexane, determined using GC-MS. Via extraction tests
with different 8 kinds of solvent, such as dichloromethane, trichloromethane,
acetonitrile, ethyl acetate, hexane-acetone (75:25), hexane-acetone (80:20),
hexane-acetone (85:15), hexane-acetone (90:10), we found that the efficiency of extraction
with dichloromethane was the best, the nine tenths recoveries were more than 90%, except
for dichlorvos (2.93%), dimethoate (40.23%). The
second was trichloromethane and ethyl acetate, the next was acetonitrile, the last was the
mixture of hexane and acetone. A half of recoveries of extraction were lower than 80% with
hexane-acetone. Moreover, the b.p. of dichloromethane was also lower than those of other
solvents, it could be used to save analytical time on the step of evaporation. Therefore,
we selected dichloromethane as the solvent of extraction.
3.3 Selection of clean-up conditions
Different proportional mixtures of hexane and acetone as eluent in the elution step, the mixture of hexane-acetone (4:1) showed the best result.
A florisil column was preconditioned under the described conditions above clean-up procedure, then pesticide standard solution was loaded onto the SPE cartridge, followed eluting with mixture of hexane-acetone (4:1) at the rate of 0.3-0.4 mL/min. Per milliliter eluent was collected respectively, till to eighth one. After concentrating and dissolving in 0.5 mL hexane, the eluent was determined by GC-MS. After the sixth milliliter, the pesticides in the eluent were almost not found, meantime, recoveries in the former 6 mL of the most pesticides were more than 85%. When the volume of eluent was more than 6 mL, the impurities could be eluted. So we selected 6 mL as volume of elution solvent.
3.4 Validations of the method
3.4.1 Accuracy and precision
The recovery rate of each pesticide at two different fortification levels was evaluated. Three repetitions of the sample were carried out for each fortification level. Quantification base on standards prepared in blank sample extract was carried out to compensate for the matrix-induced effects and to obtain more accurate results. Average recovery and relative standard deviations (RSD) obtained were shown in Table 2. TIC of blank sample and fortified sample were shown in Fig. 2 and Fig. 3, respectively.
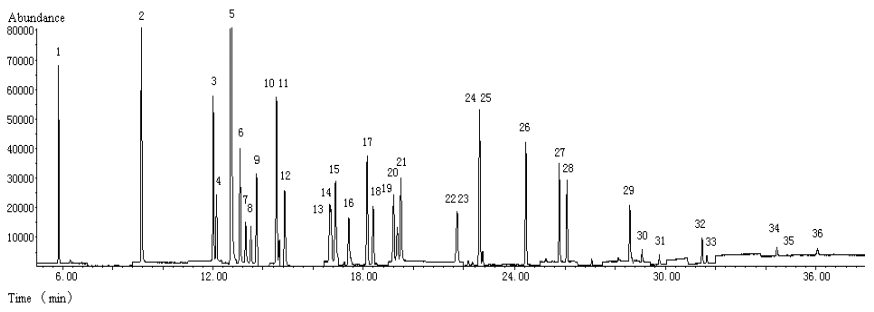
Fig. 1 TIC of the mixture solution of pesticide standards
1.dichlorvos (0.25m g/mL), 2.isoprocarb (0.25m g/mL), 3.phorate (0.20m g/mL), 4.α-BHC (0.10m g/mL), 5.dimethoate (2.0m g/mL), 6.carbofuran (0.50m g/mL), 7.β-BHC (0.10m g/mL), 8.lindane (0.10m g/mL), 9.pentachloronitrobenzene (0.50m g/mL), 10.diazinon (0.25m g/mL), 11.δ-BHC (0.10m g/mL), 12.chlorothalonil (0.25m g/mL), 13.methyl parathion (0.25m g/mL), 14.vinclozolin (0.25m g/mL), 15.carbaryl (1.0m g/mL), 16.paraoxon (0.50m g/mL), 17.fenitrothion (0.50m g/mL), 18.pirimiphos-methyl (0.10m g/mL), 19.fenthion (0.20m g/mL), 20.parathion (0.20m g/mL), 21.triadimefon (1.0m g/mL), 22.isofenphos (0.10m g/mL), 23.quinalphos (0.25m g/mL), 24.op`-DDE (0.50m g/mL), 25.endosulfan (0.50m g/mL), 26.op`-DDD (0.50m g/mL), 27.pp`-DDD (0.50m g/mL), 28.ethion (0.50m g/mL), 29.phosmet (1.0m g/mL), 30.fenpropathrin (0.25m g/mL), 31.phosalone (0.25m g/mL), 32.permethrin I (0.50m g/mL), 33.permethrin II (0.50m g/mL), 34.fenvalerate I (0.50m g/mL), 35.fenvalerate II (0.50m g/mL), 36.deltamethrin (1.0m g/mL)
Table 2 Average recovery and relative standard deviations (RSD)
No |
Compound |
Fortified level |
Recovery (%) |
RSD |
|||
I |
II |
III |
Average |
||||
1 |
dichlorvos |
0.031 |
20.94 |
18.34 |
17.70 |
18.99 |
9.03 |
0.12 |
7.83 |
6.64 |
7.56 |
7.34 |
8.50 |
||
2 |
isoprocarb |
0.031 |
88.41 |
83.28 |
80.21 |
83.97 |
4.93 |
0.12 |
91.22 |
87.35 |
87.51 |
88.69 |
2.47 |
||
3 |
phorate |
0.025 |
107.67 |
102.83 |
101.92 |
104.14 |
2.97 |
0.10 |
116.40 |
113.70 |
113.33 |
114.48 |
1.46 |
||
4 |
BHC alpha isomer |
0.012 |
120.37 |
118.77 |
117.21 |
118.78 |
1.33 |
0.050 |
114.54 |
106.58 |
112.82 |
111.31 |
3.76 |
||
5 |
dimethoate |
0.25 |
2.56 |
2.45 |
2.94 |
2.65 |
9.70 |
1.0 |
9.28 |
9.29 |
8.42 |
9.00 |
5.55 |
||
6 |
carbofuran |
0.062 |
82.63 |
70.14 |
68.93 |
73.90 |
10.26 |
0.25 |
75.16 |
68.13 |
71.48 |
71.59 |
4.91 |
||
7 |
BHC beta isomer |
0.012 |
99.74 |
98.35 |
83.05 |
93.71 |
9.88 |
0.050 |
105.73 |
100.51 |
105.05 |
103.76 |
2.73 |
||
8 |
lindane |
0.012 |
117.73 |
124.70 |
111.07 |
117.83 |
5.78 |
0.050 |
103.99 |
101.32 |
122.67 |
109.33 |
10.64 |
||
9 |
pentachloronitrobenzene |
0.062 |
95.82 |
92.18 |
90.01 |
92.67 |
3.17 |
0.25 |
115.68 |
111.29 |
113.66 |
113.54 |
1.94 |
||
10 |
diazinon |
0.031 |
96.24 |
98.22 |
96.12 |
96.86 |
1.22 |
0.12 |
115.07 |
110.94 |
113.92 |
113.31 |
1.88 |
||
11 |
BHC delta isomer |
0.012 |
99.47 |
97.84 |
90.16 |
95.82 |
5.19 |
0.050 |
99.16 |
102.99 |
105.31 |
102.49 |
3.03 |
||
12 |
chlorothalonil |
0.031 |
78.15 |
76.42 |
74.25 |
76.27 |
2.56 |
0.12 |
101.54 |
95.46 |
101.03 |
99.34 |
3.40 |
||
13 |
methyl parathion |
0.031 |
83.33 |
79.70 |
77.65 |
80.23 |
3.59 |
0.12 |
116.97 |
112.52 |
116.59 |
115.36 |
2.14 |
||
14 |
vinclozolin |
0.031 |
102.05 |
100.70 |
98.40 |
100.38 |
1.84 |
0.12 |
114.22 |
108.43 |
112.09 |
111.58 |
2.62 |
||
15 |
carbaryl |
0.12 |
67.50 |
61.03 |
55.16 |
61.23 |
10.08 |
0.50 |
65.63 |
55.15 |
60.03 |
60.27 |
8.70 |
||
16 |
paraoxon |
0.062 |
87.28 |
85.57 |
81.71 |
84.85 |
3.36 |
0.25 |
90.22 |
81.63 |
92.94 |
88.26 |
6.69 |
||
17 |
fenitrothion |
0.062 |
79.55 |
78.08 |
73.15 |
76.93 |
4.36 |
0.25 |
118.04 |
119.94 |
121.68 |
119.89 |
1.52 |
||
18 |
pirimiphos-methyl |
0.012 |
101.32 |
102.11 |
96.59 |
100.01 |
2.98 |
0.050 |
113.89 |
109.34 |
113.16 |
112.13 |
2.18 |
||
19 |
fenthion |
0.025 |
103.58 |
102.85 |
99.36 |
101.93 |
2.21 |
0.10 |
117.14 |
110.59 |
114.88 |
114.20 |
2.91 |
||
20 |
parathion |
0.025 |
84.85 |
82.02 |
77.35 |
81.41 |
4.65 |
0.10 |
117.42 |
119.41 |
116.97 |
117.93 |
1.10 |
||
21 |
triadimefon |
0.12 |
94.55 |
95.11 |
88.72 |
92.79 |
3.81 |
0.50 |
114.79 |
106.67 |
112.79 |
111.42 |
3.80 |
||
22 |
isofenphos |
0.012 |
95.67 |
93.45 |
89.31 |
92.81 |
3.48 |
0.050 |
115.87 |
109.81 |
114.15 |
113.28 |
2.76 |
||
23 |
quinalphos |
0.031 |
100.30 |
99.42 |
93.57 |
97.76 |
3.74 |
0.12 |
119.68 |
112.00 |
117.18 |
116.29 |
3.37 |
||
24 |
op`-DDE |
0.062 |
99.40 |
98.70 |
94.43 |
97.51 |
2.76 |
0.25 |
108.35 |
98.83 |
103.88 |
103.69 |
4.59 |
||
25 |
endosulfan |
0.062 |
103.95 |
104.76 |
97.45 |
102.05 |
3.93 |
0.25 |
110.35 |
101.12 |
106.39 |
105.95 |
4.37 |
||
26 |
op`-DDD |
0.062 |
104.69 |
103.27 |
97.92 |
101.96 |
3.50 |
0.25 |
113.56 |
101.41 |
108.26 |
107.74 |
5.65 |
||
27 |
pp`-DDD |
0.062 |
101.69 |
99.54 |
93.28 |
98.17 |
4.45 |
0.25 |
111.43 |
98.13 |
105.18 |
104.91 |
6.34 |
||
28 |
ethion |
0.062 |
91.49 |
88.70 |
84.29 |
88.16 |
4.12 |
0.25 |
117.99 |
105.68 |
113.48 |
112.38 |
5.54 |
||
29 |
phosmet |
0.12 |
90.46 |
91.67 |
83.89 |
88.67 |
4.72 |
0.50 |
99.41 |
91.27 |
98.01 |
96.23 |
4.52 |
||
30 |
fenpropathrin |
0.031 |
97.00 |
97.84 |
102.45 |
99.10 |
2.96 |
0.12 |
96.59 |
96.31 |
86.39 |
93.10 |
6.24 |
||
31 |
phosalone |
0.031 |
102.71 |
106.70 |
95.72 |
101.71 |
5.46 |
0.12 |
108.80 |
103.95 |
110.62 |
107.79 |
3.20 |
||
32 |
Permethrin* |
0.062 |
95.18 |
100.59 |
98.73 |
98.17 |
2.80 |
0.25 |
94.57 |
90.76 |
94.74 |
93.36 |
4.02 |
||
33 |
Fenvalerate* |
0.062 |
90.64 |
91.16 |
93.77 |
91.86 |
1.83 |
0.25 |
112.74 |
121.68 |
120.01 |
118.14 |
4.02 |
||
34 |
deltamethrin |
0.12 |
82.16 |
88.31 |
99.03 |
89.83 |
9.50 |
0.50 |
117.38 |
115.50 |
122.40 |
118.43 |
3.01 |
||
*The sum of the two isomers.
3.4.2 Linearity and the limits of detection (LOD)
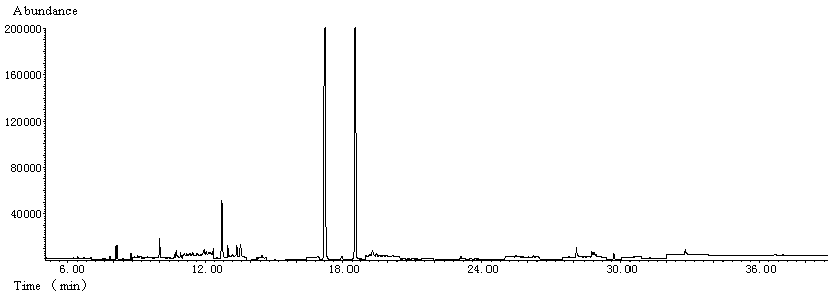
Fig. 2 TIC of blank sample
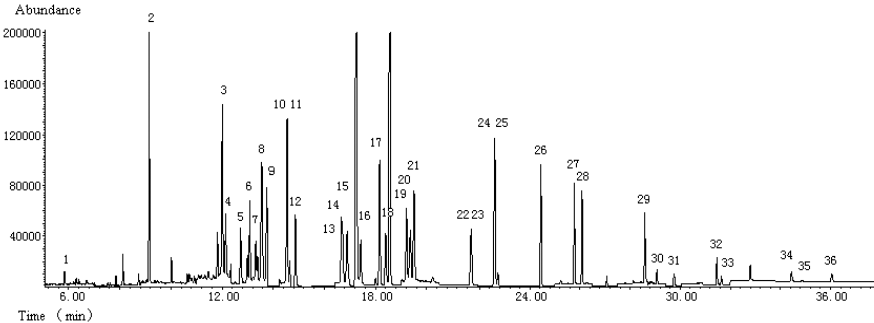
Fig. 3 TIC of fortified sample
1.dichlorvos (0.12mg/kg), 2.isoprocarb (0.12mg/kg), 3.phorate (0.10mg/kg), 4.α-BHC (0.050mg/kg), 5.dimethoate (1.0mg/kg), 6.carbofuran (0.25mg/kg), 7.β-BHC (0.050mg/kg), 8.lindane (0.050mg/kg), 9.pentachloronitrobenzene (0.25mg/kg), 10.diazinon (0.12mg/kg), 11.δ-BHC (0.050mg/kg), 12.chlorothalonil (0.12mg/kg), 13.methyl parathion (0.12mg/kg), 14.vinclozolin (0.12mg/kg), 15.carbaryl (0.50mg/kg), 16.paraoxon (0.25mg/kg), 17.fenitrothion (0.25mg/kg), 18.pirimiphos-methyl (0.050mg/kg), 19.fenthion (0.10mg/kg), 20.parathion (0.10mg/kg), 21.triadimefon (0.50mg/kg), 22.isofenphos (0.050mg/kg), 23.quinalphos (0.12mg/kg), 24.op`-DDE (0.25mg/kg), 25.endosulfan (0.25mg/kg), 26.op`-DDD (0.25mg/kg), 27.pp`-DDD (0.25mg/kg), 28.ethion (0.25mg/kg), 29.phosmet (0.50mg/kg), 30.fenpropathrin (0.12mg/kg), 31.phosalone (0.12mg/kg), 32.permethrin I (0.25mg/kg), 33.permethrin II (0.25mg/kg), 34.fenvalerate I (0.25mg/kg), 35.fenvalerate II (0.25mg/kg), 36.deltamethrin (0.50mg/kg)
4. CONCLUSION
At two different fortification levels,
all of average recovery was from 70% to 120 % except for dichlorvos, dimethoate and
carbaryl), and RSD were lower than 11% in all cases. The LOD were between 0.0013 mg/kg and
0.065 mg/kg, these values were lower then MRLs regulated by Europe Union (EU, 0.01-5mg/kg) and Codex
Alimentarius Commission (CAC, 0.02-0.1mg/kg)
[16].
This method
was simple, rapid, sensitive and could simultaneously determine multicomponent pesticide
residues in corn, and satisfied the requirement of routine analysis of pesticide residues
in corn.
The recoveries of dichlorvos, dimethoate and carbaryl were lower. It
could have two reasons: one is these pesticides are very well water-solubility, extract
these pesticides from water using dichloromethane is difficult, another is that the
florisil absorbent is of somewhat polarity, it will adsorb these pesticides in the
clean-up procedure.
ACKNOWLEGEMENTS
The authors acknowledge Hebei Science and Technology Department, and Science and
Technology Bureau, of Baoding city. (Project: 03547023D, 03N006)
[1]Frank J S, Vinetta H K, Journal of Environmental Science and Health B, 2000, 35 (1) : 1-12.
[2]Tomoko I, Saori S, Seili U, et al. Journal of Food Hygienic Society of Japan, 2003, 44 (6): 310-315.
[3] Liu A J, Yin D L, Jour Nat Scie Hunan Norm Uni( Hunan Shifan Daxue Ziran kexue Xuebao), 2001, 24 (1): 52-54.
[4] Long S, Zhou Y G, Wang X C, et al. Practical Preventive Medicine (Shiyong Yufang Yixue), 2001, 18 (5): 341-342.
[5] Duan T T, Liao G H, Guizhou Agricultural Sciences( Guizhou Nongye Kexue), 2002, 30 (3): 61-62.
[6] Saikia N, Kulshrestha G, Journal of Chromatography A, 1999, 864: 351–353.
[7] Lin W X, Tian M, Li J Y, et al. Journal of the Chinese Cereals and Oils Association( Zhongguo Liangyou Xuebao) , 2003, 18 (2): 79-84.
[8] Dai H, Li Y J, Zhang Y, Journal of Instrumental Analysis( Fenxi Ceshi Xuebao), 2002, 21 (1): 70-72.
[9] Hogendoorn E A , Ossendrijver F M, Dijkman E, et al. Journal of Chromatography A, 1999, 833: 67-73.
[10] Li Y J, Huang Z Q, Yi W L, Chinese Journal of Chromatography (Sepu), 2002, 20 (2): 190-192.
[11] Liu C W, Liu F Z, Zhai G S, et al. Acta Scientiae Circumstantiae( Huanjing Kexue Xuebao), 2003, 23 (4): 499-502.
[12] Lino C M, Silveira I N, Journal of Chromatography A, 1997, 769: 275-283.
[13] Jimenez J J, Bernal J L, Noza M.J, et al, Journal of Chromatography A, 2001, 919: 147–156.
[14] Bernal J L., Jimenez J J, Nozal M J, et al. Journal of Chromatography A, 2000, 882: 239–243.
[15] Burke E R, Holden A J, Shaw I C, Chemosphere, 2003, 50: 529-535.
[16] Lin W X, The Compilation of Residue Limits Standards for Pesticides and Veterinary Drugs in Foodstuffs in the World( Geguo Shipin Zhong Nongyao Shouyao Canliu Xianliang Guiding), Dalian: Dalian Maritime University Press, 2002: 3-95.
气相色谱—质谱联用测定玉米中36种农药残留量
刘芃岩 马育松
( 河北大学 化学与环境科学学院,河北 保定 071002)
摘要 建立了玉米中有机磷、有机氯、氨基甲酸酯和拟除虫菊酯等多种类农药残留量的气质联用的测定方法。本方法采用二氯甲烷提取、Florisil固相萃取小柱净化,以保留时间和选择离子比例定性,以空白样品添加为标准进行定量分析,通过方法的准确度、精密度、检出限的测定结果证明了方法的可靠性。
关键词 气质联用;农药;多残留;玉米