http://www.chemistrymag.org/cji/2005/078054ne.htm |
Aug. 12, 2005 Vol.7 No.8 P.54 Copyright |
Enantioselective transport of optical isomers via chiral polysaccharide membrane
Xiao Dingshu 1, Hu Jiwen1,2,*, Zhang Mingqiu2, Li Mingwei1, Feng Liang1(1Key Laboratory of Polymer Materials for Electronics of Guangdong Province, Guangzhou Institute of Chemistry, Chinese Academy of Sciences, Guangzhou 510650; 2Key Laboratory for Polymeric Composite and Functional Materials of Ministry of Education, Zhongshan University, Guangzhou 510275) Abstract In this work crosslinked sodium alginate (SA) membrane was prepared and its enantioselective transport of a-amino acids was studied. It was found that the resultant thin membrane exhibits high permeability coupled with encouraging enantioselectivity. Low temperature helps to enhance the enantioselectivity but leads to reduced permeation rate. The different enantioselective transports of tyrosine and phenylalanine enantiomers through the chiral membranes confirmed the significance of hydrogen bond in enantioseparation.
Keywords Sodium alginate, membrane, enantioselective, hydrogen bond.
Techniques for preparative-scale enantiomer
resolution are urgently needed because of the growing interests of the pharmaceutical
industry in single-enantiomer drugs. The newly developed resolution procedures based on
enantioselective polymeric membranes, characterized by their continuous operation ability,
convenient up-scaling and energy-saving fashion, have been recognized as the most
challenging and attractive way for purification of chiral drugs and natural products[1].
The optical resolution through enantioselective membranes is right at the beginning stage.
The efficiency, enantioselectivity and stability, controlled by many factors (e.g.,
structure parameters of the polymer, interactions between the chiral
environment and enantiomers, size and shape of chiral penetrant molecules), are not high
enough for practical usage[2-4]. Therefore, a comprehensive understanding of
the enantioselective transport behavior is necessary for designing highly efficient and
economically viable membranes.
Sodium alginate (SA), comprised of linear chains of 1,4-linked b
1 EXPERIMENTAL
Commercial sodium alginate (analytical grade, Sigma-Aldrich Co.) was applied for the
formation of enantioselective membranes. The coupling aqueous solution, containing
dissolved SA at 40
Permeation tests were carried out in the way similar to the procedures reported in the literature[5]. The membrane is placed between two chambers in water bath at a constant temperature. Tyrosine or phenylalanine (biochemical grade, HuiXing Biochemical Co. Ltd., China) solution (500 ml, 1.5 mM) was poured into one of the chambers and the solvent (500 ml de-ionized water) into the other. Permeation rate (P) was estimated from the permeated quantity (q) in single-enantiomer permeation, which was obtained by monitoring concentration using SP-2100 UV-visible spectrophotometer at maximum absorbance 272 nm and 253 nm for tyrosine and phenylalanine, respectively.
P (g·m·m-2·h-1) = (q×L) / (A×t)
where t denotes the permeation time, L and A the thickness and area of the membrane, respectively. Enantiomeric excess (% e.e.) and separation factor (a) were obtained from the permeated quantity in racemic permeation, which was reckoned by the concentration of the permeated solution and its specific rotation measured by a Model W22-1S automatic polarimeter.
% e.e. = (qD - qL) / (qD + qL) × 100% a= qD / qL
where qD and qL are the isomer permeated quantities in recemic permeation, respectively.
2 RESULTS AND DISCUSSION
Table 1 shows the membrane formation ability and membrane quality at various SA
concentrations in the solution. It can be seen that the membranes 10-15 m
So far, it is known that improvement of permeability is of the same importance as that of enantioselectivity, as the former determines the scale and efficiency of the given enantiomers resolution. According to the mechanism of transport, there are lots of routes for enhancing permeability including the application of host membranes with high porosities, permeation under high pressure and / or temperature, reduction of membrane thickness[3]. However, chiral polymeric membranes with both high enantioselectivity and permeability are difficult to be made[4]. Table 1 Effect of SA concentration on membrane formation ability and membrane quality
SA
concen-tration |
Membrane formation ability and surface flatness |
Thickness |
Peeling ability |
Tensile strength (MPa) |
0.5 |
Unable to form membrane |
---- |
----- |
----- |
1.0 |
Good, uneven, partly wrinkled |
5-8 |
Difficult |
11-15 |
1.5 |
Good and easy, even, not wrinkled |
10-15 |
Easy |
17-20 |
5.0 |
Good but difficult, uneven, not wrinkled |
50-60 |
Easy |
19-21 |
The data in Figure 1 and Table 2 were obtained from the SA membrane with a thickness of 10-15 m
m. It is seen that the fluxes of racemate are approximately half of the values of the two neat enantiomers. In addition, the separation factors coincide with the ratios of the two permeation rates in single-enantiomer permeation test. These results suggest that the separation factor can be converted into the permeation rate ratio (a= PD/PL) in the single-enantiomer permeation. Analogously, the permeation rate yielded from single-enantiomer permeation test can also represent the isomer transport in racemic permeation. The high L-phe flux clearly reveals the enantioselective trend of L-phenylalanine transport. In contrast, a contrary enantioselective pattern (i.e., the permeation rate of L-Tyr is lower than that of D-Tyr), is observed in Figure 1b, probably due to the different enantiorecognition mechanisms[6].As expected, the flux increases with decreasing membrane thickness. The permeability rate of tyrosine of the current SA membrane is about 10 times higher than that measured under a pressure of 1-2 ㎏f/cm2 as reported in the literature[2], indicating that it is an effective way to improve the permeating rate by decreasing membrane thickness. What is more, it is interesting to find that the enantioselectivity can also be enhanced simultaneously. The results show that the values of %e.e. and a are 33.56 and 2.01 for the membrane, respectively. They are much higher than those reported in the literature[2]. Therefore, the mechanism involved in the permeation behavior of the enantioselective membrane cannot be simply explained by the common model like solution-diffusion one, and need to be studied further.
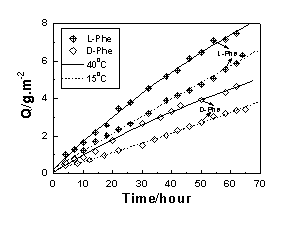
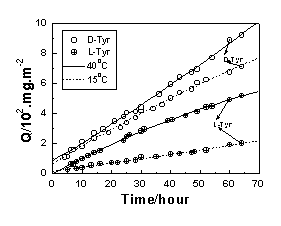
Figure 1 Plots of the amount of the permeant permeated through the membrane versus time at 25 ºC and 40ºC. (a) phenylalanine, and (b) tyrosine
Table 2 Enantioselective permeation of amino acids through the SA membrane
Temp. |
Phenylalanine |
Tyrosine |
||||||
| P ×10-5 g·m·m-2·h-1 |
ratio |
a | %e.e. |
P ×10-6 |
ratio |
a | %e.e. |
|
15 |
0.79-1.34 |
1.69 |
1.57 |
22.17 |
0.68-1.45 |
2.13 |
2.01 |
33.56 |
40 |
1.07-1.63 |
1.52 |
1.42 |
17.36 |
1.02-1.82 |
1.78 |
1.67 |
25.09 |
Basically,
enantiorecognition and enantioselective transport can be achieved through the interaction
of enantiomers with chiral environment in the membrane. In the case of SA membrane, the
chiral environment is assembled by the chiral small and larger spacers based on chiral
helical structures on the ring backbones, and thus contributes to the enantioselective
recognition[2]. Herein, the hydrogen bonding plays the leading role in the
transport behavior. The statement is proved by the different transports of Tyr and Phe
enantiomers that have similar molecular structures except the phenolic hydroxyl group.
When the other conditions are equal, lower transfer rate, higher enantiomer excess value
and separation factor of Tyr enantiomers than those of Phe enantiomers are mainly
attributed to the strong affinity of chiral membrane to Tyr (see Table 2).
On the other hand, Figure 1 shows that more obvious
enantioselective trend and lower permeation rate are recorded at lower temperature. This
is due to the fact that the fast molecular movement induced by elevated temperature might
result in weakened interaction between the chiral sites. Figure 1 also illustrates
that the changes of selectivity and permeation rate of Tyr are more sensitive than those
of Phe. A possible explanation for this phenomenon might originate from the different
chiral recognition mechanisms. That is, the low temperature enantioselectivity is governed
by entropy due to inclusion, whereas the high temperature enantioselectivity by the
enthalpy of hydrogen bonding[6, 7].
Acknowledgment The financial support by the National Natural Science Foundation of China (Grant: 20474068) and the Natural Science Foundation of Guangdong Province (Grants: 021471) are gratefully acknowledged.
REFERENCES
[1] Norbert N M, Franco P, Lindner W. J. Chromatogr. A, 2001, 906: 3.
[2] Jang H K, Jee H K, Jonggeon J et al. J. Membr. Sci., 2003, 213: 273
[3] Rmaile H H, Schlenoff J B, J. Am. Chem. Soc., 2003, 125: 6602.
[4] Van der ent E M, Van’t Riet K, Keurentjes J T F
et al., J. Membr. Sci., 2001, 185: 207.
[5] Nakagawa T, Toyokawa Y, Abe M et al., Macromol. Symp., 1994, 84: 209.
[6] Kano K J. Phys. Org. Chem., 1997, 10: 286.
[7] Brien T O, Crocker L, Thompson R et al. Anal. Chem., 1997, 69: 1999.
手性多糖膜对光学异构体的对映体选择传递
肖定书1, 胡继文1,2,*, 章明秋2,
李明威1, 冯良1
(1中国科学院广州化学所,广东省电子高分子材料重点实验室,广州,510650;2中山大学教育部高分子复合与功能材料重点实验室,广州,510275)
摘要
本工作制备了交联的海藻酸钠膜,并研究了该膜对a-氨基酸对映体的选择性传递行为。结果显示:所制备的薄膜具有较高的渗透能力和较理想的选择性。此外,低温有利于提高选择性,但渗透率下降。手性膜对酪氨酸和苯丙氨酸的对映体选择性传递行为的差异揭示了氢键作用在手性分离中的意义。
关键词 海藻酸钠;膜;对映体选择;氢键