http://www.chemistrymag.org/cji/2005/078053ne.htm |
Aug. 2, 2005 Vol.7 No.8 P.53 Copyright |
Analyzing photodegradation intermediates of phenol and degradation mechanism
Guo Zhifeng, Ma Ruixin, Li Guojun(Research Center of Physics and Chemistry Analysis, Hebei University; Hebei Province Key Lab of Analytical Science and Technology, Baoding, 071002) Received on Apr.30, 2005. Abstract The degradation of 100 mg/L phenol solution under ultraviolet (UV) either in the presence of or in the absence of TiO2 were analyzed with gas chromatography / mass spectrometry (GC-MS) and high performance liquid chromatography (HPLC). After 12h of degradation, the products under the two conditions were both derivatized by MSTFA and TMIS, and then analyzed by GC-MS. The results show that the main intermediates are the same. They are hydroquinone, resorcinol, catechol, 1,2,3-benzenetriol, (E)-2-butenedioic acid,2-hydroxy- propaldehyde, glycerol, 3-hydroxy-propyl acid, and hydroxy-acetic acid. This shows the photodegradation is a process including oxidation and reduction and TiO2 is not in favor of the degradation when the concentration of phenol solution is 100mg/L, its catalysis becomes evident when the concentration gets lower.
Keywords phenol; titanium dioxide; photodegradation; mechanism In industry and daily life, phenolic compounds are widely used. As they have high stability, high toxicity and a carcinogenic character, it may cause considerable damage and threat to the ecosystem in water bodies and human health. How to eliminate phenolics in the wastewater effectively has been in urgent demand. Traditional wastewater treatment techniques include activated carbon adsorption, chemical oxidation and biological digestion, in which photocatalytic degradation assisted by titanium dioxide under ultraviolet irradiation is one of the technologies studied widely. More researches have been focused on the types and modification of the catalysts, the photocatalytic principles, the factors influencing photocatalytic rate and the reaction kinetics [1-8]. The key to speculate the reaction route is to detect the intermediates. The most widely used methods to detect them are extraction by organic solvent, after esterification or acetylation, followed by analysis by gas chromatography or gas chromatography / mass spectrometry (GC-MS) [9,10]. But these methods have two shortages: (1) as the intermediates are mostly polar compounds, it is hard to separate them from water. (2) esterification only fits for derivatizing acids, while acetylation only for hydroxylated compounds. In addition to that, it is possible to detect intermediates by means of HPLC [11, 12], using retention time to analyze qualitatively. However, this method needs many calibration standards.
The aim of this work is to detect intermediates, using gas chromatography / mass spectrometry after derivatization by N-methyl-N (tri-methylsilyl)trifluoroacetamide (MSTFA) and trimethyliodosilane (TMIS), this silane reageant can derivatize organic substances with active hydrogen. Before the derivatization, no extraction was carried out; the solution after illumination was dried by nitrogen in order not to lose any substance and intermediates such as alcohols, aldehyes, acids etc. The degradations in the presence of nanomaterial titanium dioxide (TiO2) and that in the absence of TiO2 were also compared. 1 EXPERIMENTAL
Phenol and chloroform used in this experiment are analytical reagent grade. Acetic acid and methanol are of chromatographic purity. TiO2 is mixed crystal and particle size is 20-30nm, provided by Hebei University nanomaterial research center. MSTFA and TMIS were purchased from Sigma. The water used for the photocatalytic studies was doubly distilled in quartz water still.
Photocatalytic experiments were carried out in a quartz glass photoreactor, with the penetration is 69% at wavelength 253.7 nm. 40㎝3 of 100mg/L phenol solution and 8.16mg of TiO2 ( sonicated for 5 min before illumination) were illuminated with a ultraviolet lamp (20W, 253.7nm). The reactant was mixed by a magnetic stirrer; and the reaction temperature was 15±1ºC. 1㎝3 of the slurry was withdrawn respectively at the time of 0h, 3h,12h. After centrifugation, 25mL of filtrate was analyzed by HPLC (Shimadzu) equipped with a SPD-6AV detector at 280nm, A separation column was a Hypersil ODS C18 (25 cm×4.6 mm×5 mm). The mobile phase was 1% acetic acid +35% methanol + 64% water (v/v).
GC-MS was performed as follows. Primarily ,after 12h of illumination, 6㎝3 of the slurry was withdrawn from the reactor. After centrifugation, the pellucid solution was dried by nitrogen, derivatized by 50mL MSTFA and 0.5mL TMIS at 62ºC for 70min. Then 1mL was analyzed on the gas chromatography (Hewlett- Packard model 6890 series) coupled with mass spectrometry (VG70E-HF, Micromass Corporation) in scan mode between 30 and 600 m/z. The analyzed products were separated on a HP-5 column (30m×0.32mm,0.25mm) using programmed temperature: started at 28ºC, holding for 2min then to 280ºC, holding for10min at a rate of 6ºC/min, the column pressure was kept at 6.9×103Pa. The carrier gas (Helium) was kept at a flow rate of 1.5mL/min and a linear speed of 44 cm/s. The sample was injected in splitless mode. For MS analysis, ionization energy 70eV and accelerated voltage 6kV were applied; the resolving power was 1000.
40㎝3 of 100mg/L phenol solution without TiO2 was illuminated under the same conditions and analyzed with the same method, but without centrifugation. Simple extractions of the reaction products (by chloroform, without derivatization) from the solution after illumination for 3h and 12h under the two conditions were also done and analyzed by GC-MS. Because the background produced by the derivatization is complex, 6mL doubly distilled water was used as blank test with the same method. 2 RESULTS AND DISCUSSION
The main task of the study was to detect the intermediate products; 100mg/L of phenol solution was used so that the intermediates were high enough to be detected. After 3h of degradation the color of the solution became light yellow; after 12h, the color was yellow. Simple extractions of the reaction products in the presence of TiO2 or in its absence were both analyzed by GC-MS respectively, but there was no other peak except phenol in the total ion current graph, this is because chloroform did not extract the intermediates from water. The pellucid solutions after illumination under the two conditions were analyzed by HPLC; the results revealed phenol was decreased, contrasting to the content of phenol by HPLC before illumination. After derivatization by MSTFA and TMIS, the reaction products were analyzed by GC-MS; there were many peaks in the total ion current graph under both two conditions. The total ion current graphs were similar, see Fig.1.These peaks were identified by MS, The results indicated the main intermediates are the same in the two cases, they are 2-hydroxy-propaldehyde, hydroxy-acetic acid, 3- hydroxy-propyl acid, glycerol, catechol, (E)-2-butenedioic acid, resorcinol, hydroquinone and 1, 2, 3-benzenetriol. The mass spectrum of some products after derivatization by MSTFA and TMIS is reported in Fig.2. In the two ion current graphs, peak 796 in Fig.1a and 785 in Fig.1b are the sum of resorcinol and hydroquinone, since GC does not separate them and they are also very similar on the MS. There are also some high peaks in the total ion current graphs without number because they are the peaks in the background. Some small peaks could not be analyzed qualitatively because the MS information was not sufficient.
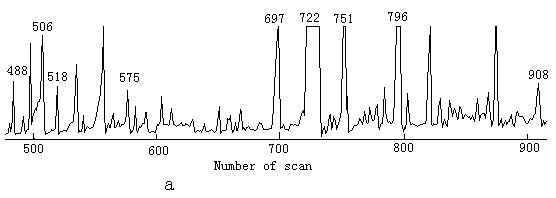
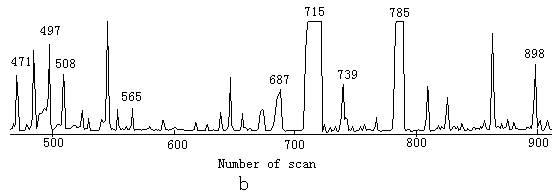
Fig.1 Total ion current graph of intermediates after 12h of ultraviolet illumination
a .in the presence TiO2; b. in the absence of TiO2
The peaks in the graph corresponding to the organic compounds before derivatization are respectively:
a 488 phenol 506 2-hydroxy-propaldehyde 518 hydroxy-acetic acid 575 3- hydroxy-propyl acid 697 glycerol 722 catechol 751 (E)-2-butenedioic acid 796 resorcinol and hydroquinone 908 1,2,3-benzenetriol
b 471 phenol 497 2-hydroxy-propaldehyde 508 hydroxy-acetic acid 565 3- hydroxy-propyl acid 687 glycerol 715 catechol 739 (E)-2-butenedioic acid 785 resorcinol and hydroquinone 898 1,2,3-benzenetriol
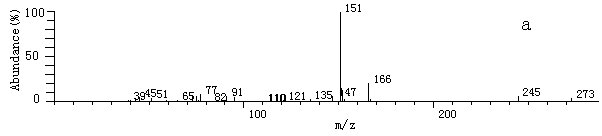
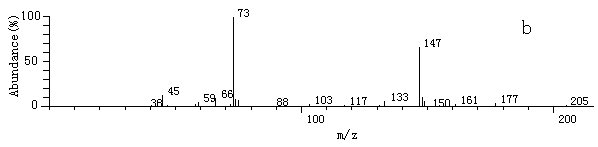

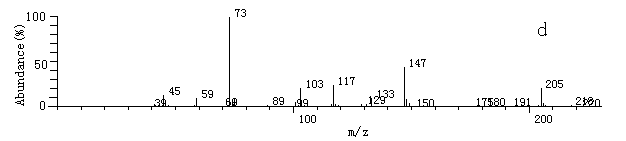
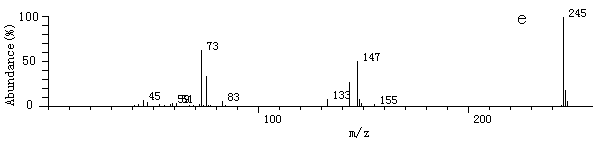 Fig.2 The
mass spectrum of some products after derivatization by MSTFA and TMIS
Fig.2 The
mass spectrum of some products after derivatization by MSTFA and TMISa. 2-hydroxy-propaldehyde; b. hydroxy-acetic acid; c. 3- hydroxy-propyl acid; d. glycerol; e. (E)-2-butenedioic acid In this experiment, hydroxyaldehyde, hydroxyacid, (E)-2-butenedioic acid, glycerol et al. were detected. Benzoquinone, maleic acid, malonic acid, some low molecular weight organic acids such as oxalic acid and acetic acid were not detected [10, 11, 13]. About the mechanism of the photodegradation by TiO2, it has been reported [4, 14]. But based on the intermediates detected by this experiment, it can be concluded that during the oxidation process, there is also reduction process happened [16, 17].
HPLC was used to analyze the degradation efficiency. The degradation efficiency of the solution in the presence of TiO2 is 16% and 76% when illuminated for 3h and 12h according to the peak areas, while the efficiency in the absence of TiO2 is 40% and 81.6% respectively. It can be seen that in the first three hours, the concentration of phenol is higher; the degradarion efficiency without TiO2 is two times more than that in presence of TiO2. As the reaction follows, the phenol concentration becomes lower and lower, the degradation efficiency in the presence of TiO2 becomes closer and closer to that without TiO2. After 12h irradation they are almost the same. This shows TiO2 decreases the reaction rate when the phenol concentration is higher; its catalysis becomes evident when the concentration is lower. Actually, the photocatalytic degradation rate of phenol in the presence of TiO2 does not increase linearly with phenol concentration. On the contrary, the rate reaches a maximum around 5×10-4M phenol and starts decreasing [15]. It is possible to account for such a trend upon consideration of the kinetics of the process taking place at the water-semiconductor interface. A substrate S can be oxidized upon reaction with holes or hydroxyl radicals on photocatalyst surface, but the oxidized intermediate can react with reducing species (e.g. electrons) yielding back S, which finally results in a decrease of the degradation rate of the substrate (phenol, but also other compounds) with increasing initial concentration [15-17]. A simplified scheme of the overall process is reported below (oxidizing and reducing species are generically indicates as h+ and e-):
TiO2 + hv → h+ + e-
S + h+ →
3 CONCLUSION
The main intermediates of phenol degradation under ultraviolet illumination are the same, whether nanomaterial TiO2 is present or not. They include 2-hydroxy-propaldehyde, hydroxy-acetic acid, 3- hydroxy-propyl acid, glycerol, catechol, (E)-2-butenedioic acid, resorcinol, hydroquinone and 1, 2, 3-benzenetriol. When the concentration of phenol is 100mg/L, TiO2 does not favor the degradation; as the concentration gets lower, its catalysis becomes obvious step by step. ACKNOWLEDGEMENTS We would like to thank the personal in Prof. Shiwen Ding's Nanomaterial research lab for providing Nanomaterial TiO2. REFERENCES
[1] Zhang J C, Gao L , Cao W L. Chinese Journal of Inorganic Chemistry, 2003, 19 (9): 934-940.
[2] Wong K H., Tao S, Dawson R et al. Journal of Hazardous Materials B, 2004, 109:149-155.
[3] Cheng S F ,Tsai SJ, Lee Y F. Catalysis Today, 1995, 26: 87-96.
[4] Wang K H, Hsieh Y H, Chou M Y et al . Applied Catalysis B: Environ, 1999, 21: 1-8.
[5] Fortuny A, Bengoa C,.Font J et al. Cataysis Today, 1999, 53: 107-114.
[6] Maugans C B, Akgerman A. Water Research, 1997, 31:3116-3124.
[7] Bhatkhande D S, Kamble S P, Sawant S B. Chemical Engineering Journal, 2004,102:283-290.
[8] Hu C, Wang Y Z, Tang H X. Environmental Science, 1997, 18: 1-5.
[ 9 ] Sobczyński A, Duczmal L, Zmudziński W. Journal of Molecular Catalysis A: Chemical, 2004, 213: 225-230.
[10] Miao X S, Chu S G, Xu X B et al. Environmental Chemistry, 1995, 14: 436-441.
[11] Alnaizy R, Akgerman A. Advances in Environmental Research, 2000, 4: 233-244.
[12] Drenzek N J , Nyman M C , Clesceri N L et al. Chemosphere, 2004, 54: 387-395.
[13] Wang Y Z, Hu C, Tong H X. Acta Scientiae Circumstantiae, 1995, 15 (4): 473-479.
[14] Wu C D, Liu X H, Wei D B et al. Water Research, 2001, 35: 3927-3933.
[15] Vione D, Minero C. Maurno V et al. Applied catalysis B: Environmental, 2005, 58: 81-90.
[16] Minero C. Catalysis Today, 1999, 54: 205-216.
[17] Minero C, Mariella G, Maurino V et al. Langmuir, 2000, 16: 2632-2641.
苯酚光氧化降解过程中的中间体及其降解机理的分析
郭志峰 马瑞欣 李国俊
(河北大学理化分析研究中心,河北省分析科学技术重点试验室,保定
071002)
摘要 本文采用气相色谱-质谱联用仪(GC-MS)和高效液相色谱(HPLC)分析手段,研究了苯酚水溶液添加和不添加二氧化钛(TiO2)的紫外降解率和中间产物。降解产物进行了硅烷化衍生,与酯化和乙酰化衍生比较,检测出了更多的产物。GC-MS分析结果表明两种情况下苯酚降解的主要产物相同,有对苯二酚、间苯二酚、邻苯二酚
、1,2,3-苯三酚、反-丁烯二酸、2-羟基丙醛、丙三醇、3-羟基丙酸、羟基乙酸。高效液相色谱(HPLC)分析结果发现:苯酚浓度100g/ml时,TiO2的存在不利于降解的进行;随着浓度降低,
TiO2的光催化作用显现。根据检测到的降解产物推知,苯酚紫外降解是一个既有氧化又有还原的过程,降解过程中,碳链逐步变短,最后生成CO2。
关键词 苯酚; 二氧化钛; 紫外光降解; GC-MS;
降解反应机理