http://www.chemistrymag.org/cji/2005/07a069pe.htm |
Oct. 12, 2005 Vol.7 No.10 P.69 Copyright |
Ning Qiaoyu, Meng Jianxin, Wang Haiming
(Department of Chemistry, Jinan University, Guangzhou 510632, China)
Received Aug. 10, 2005; Supported by the National Science Foundation of China (No. 20475021)
Abstract In an effort to prepare
efficient and uniform biolabel, a kind of novel fluorophore doped silica nanoparticles via
water-in-oil (W/O) microemulsion method has been developed. 5-aminofluorescein (AMF) was
connected to 3-aminopropyltriethyloxysilane (APTEOS) with dianhydride of
diethylenetriaminepentaacetic acid (DTPAA) to form a monomer fluorophore silica precursor.
The controlled co-hydrolysis of tetraethyl orthosilicate (TEOS) and the precursor in W/O
microemulsion leads to the formation of monodisperse fluorophore doped silica
nanoparticles. The transmission electron microscope (TEM) photograph showed that the
particles are very uniform and the fluorophores are dispersed homogeneously throughout the
silica network, with a diameter of about 40nm.The nanoparticles have shown several unique
advantages over the previous nano-biolabel in easy preparation, good photostability and
significantly reduced fluorescent dye leaching. The particles are potential of good
biocompatibility, since they have a silica surface and can thus be modified easily and
connected to various biomolecules for added biochemical functionality.
Keywords 5-aminofluorescein, microemulsion, fluorescent nanoparticles
1 INTRODUTION
Recently, molecular probes for bimolecular recognition are of great importance in the
fields of chemistry, biology and medical science as well as in biotechnology. Fluorescent
labeling of biological materials using small organic dyes has been widely employed in the
life science including diagnostics and biological imaging [1]. Problems arise
mainly from poor photostability and brightness, especially for samples with high
background fluorescence [2]. Therefore, it is highly desirable to develop new
fluorescent probes for biochemical assays. Fluorophore doped nanoparticles have draw much
more attention recently [3,4] since the fluorophores doped inside the
nanoparticles are well protected from the surrounding environments and are, thus, immune
to the potential quenching and bleaching in solution. Recently, Yang et al [5] reported the use of lissamine rhodamine B
sulfonylchloride hybrid silica nanoparticles in the sandwich-model immunoassay for the
detection of trace level HBsAg. They coupled the dye fluorescein isothiocyanate (FITC)
into the silica spheres in a quite rigorous condition because the organic dye molecules
could not be easily doped into the silica matrix. One of the reasons is that many dyes are
hydrophobic, which will be quickly partitioned to the organic phase during the
polymerization of tetraethyl orthosilicate (TEOS). Even for highly water-soluble dyes, a
serious dye leakage from the silica matrix after the dispersion in an aqueous solution
still exists if there are no retaining forces between dye molecules and the silica matrix [6]. It has been reported the dye leaching can be reduced if
the dye has been connected to IgG, a Y shape protein [7], but IgG is very
expensive, which leads to limit in practice.
In the present work, AMF was coupled with DTPAA and APTEOS. By
synthesizing a monomer fluorophore silica precursor of 5-aminofluorescein (AMF), we have
successfully prepared fluorescent hybrid silica nanoparticles via water-in-oil (W/O)
microemulsion method. The particles have uniform size, good photostability, good
biocompatibility, and high quantum efficiency. Furthermore, it can significantly reduce
fluorescent dye leaching from the nanoparticles to use the precursor as core material,
since DTPAA is a dianhydride compound with polycarboxyl that leads to the high stability
of the precursor. The silica shell surface can be further functionalized in numerous ways
to meet different needs.
2 EXPERIMENTAL
2.1 Materials and apparatus
5-aminofluorescein (AMF, Acros), 3-aminopropyltriethyloxysilane (APTEOS, Acros),
tetraethyl orthosilicate (TEOS, Aldrich), dimethylsulfoxide (DMSO), Triton X-100,
n-hexanol, and cyclehexane, ammonium hydroxide (28-30wt %) were used as received,
dianhydride of diethylenetriaminepentaacetic acid (DTPAA) prepared as the reference [8],
Distilled water was used throughout.
Fluorescence was determinated with a Hitachi F-4500 fluorescence
spectrophotometer. Size and shape of the nanoparticles were characterized with Philip
Tecnai-10 transmission electron microscope.
2.2 preparation methods
2.2.1 Synthesis of monomer fluorophore silica precursor
Monomer fluorophore silica precursor, AMF-DTPAA-APTEOS (abbreviate as ADA), was
prepared by the following procedure:
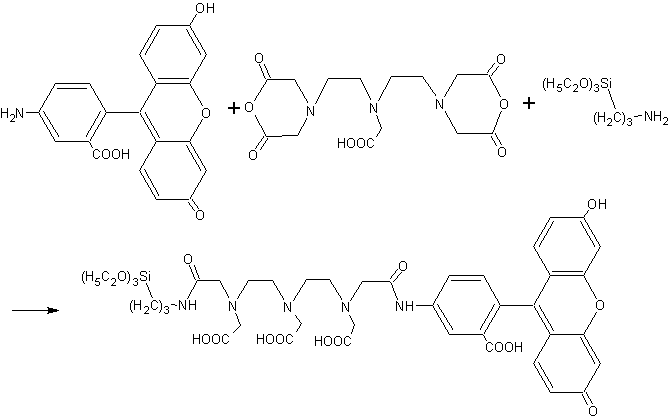
DTPAA (0.14mmol) was dissolved in 2ml
DMSO, and then AMF (0.14mmol) was added with stirring, after stirring for 8h, 33mL APTEOS (0.14mmol) was added. The
mixture was allowed to react overnight at ambient temperature. Since the byproduct did not
affect the following procedure and could be easily removed after formation of the
nanoparticles, the monomer precursor was not further isolated.
2.2.2 Preparation of nanoparticles
A water-in-oil (W/O) microemulsion [9] containing 2.3mLof Triton X-100,
2.3mL of n-hexanol, 9.3mL of cyclohexane, 200mL of TEOS, and 1.1mL of an aqueous solution of 200mL monomer precursor
was mixed with a W/O microemulsion containing 2.3mLof Triton X-100, 2.3mL of n-hexanol,
9.3mL of cyclohexane, 200mL of concentrated ammonium hydroxide with vigorous stirring. After
stirring for 24h, the precipitation was obtained by adding acetone, centrifuging,and repeatedly washing with ethanol and water to remove surfactant
and unreacted materials. The obtained fluorescent nanoparticles were dried in a vacuum
desiccator for the following use.
2.2.3 Dye leaching experiment
Appropriate nanoparticles were added in 4mL water with ultrasonic for 15min to obtain
a suspension. Fluorescence intensity of the suspension was determined. Then the suspension
was centrifuged and the precipitate was dispersed in 4mL water again. The leaching extent
of organic dye could be understood from the change of fluorescence intensity with
repeating of the disperse and centrifuge processes
3 RESULTS AND DISCUSSION
3.1 The preparation of nanoparticles
The nanoparticles have been prepared by the water-in-oil microemulsion method. A
controlled co-hydrolysis of TEOS and the monomer precursor in W/O microemulsion has led to
the formation of monodisperse ADA-doped silica nanoparticles. The ADA-doped nanoparticles
are uniform in size, 40±5nm in diameter, as characterized by TEM (Fig.1).
Fluorescence spectrometer measurements were also used to characterize
the nanoparticles. The excitation and the emission spectra of AMF, ADA monomer precursor
and ADA-doped nanoparticles were measured in aqueous solution; AMF shows emission at 519nm
when excited at 496nm excitation band maxima (Fig.2). There is a blue shift of 6nm in
excitation spectra of ADA compared with that of AMF, while ADA-doped nanoparticles have a
red shift. The blue shift of ADA may be due to the formation of chemical bond between AMF
and DTPAA, while the red shift of nanoparticles may be due to the presence of silica
network surrounding the fluorophores.
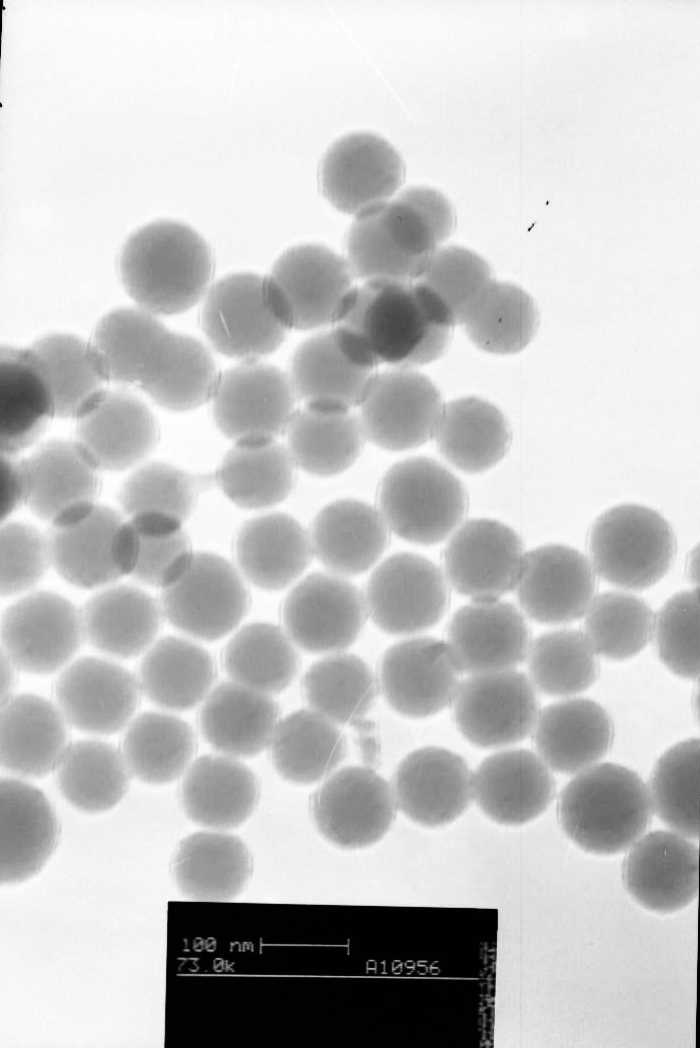
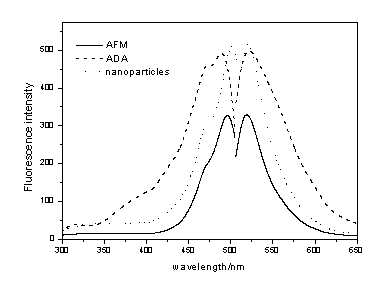
Fig.1 TEM image of ADA-dope nanopartiles |
Fig.2 Excitation and emission spectrum |
3.2 Dye leaching experiment
To date, most of the fluorescent material that has been used to prepare core-shell
nanoparticles is limited in metal coordination compound; while organic fluorescent labels
that have higher quantum efficiency have not been widely used in the preparation of
core-shell nanoparticles. Experiments show that using traditional method of preparing
core-shell nanoparticles to prepare organic dye doped core-shell nanoparticles induce
remarkable fluorescent dye leakage. Dye leakage could be reduced by connecting the dye
molecule to some protein with unique shape, such as IgG. Because IgG has a "Y"
shape organized protein structure, which can increase the
steric hindrance and protected the dye leaching from the nanoparticles. However, IgG is
very expensive, which leads to limit in practice. IgG is also prone to change its shape
after denaturalization which may cause addition leakage of the dye. In this paper, forming
chemical bond among AFM, DTPAA, APTEOS and the silica
framework prevented leakage of the fluorescein. It is obvious that the present method is
more stable and much less expensive; the result is shown in Table 1. After washing for 9
times, the fluorescence intensity dropped only 5% of the initial intensity, which may
result from the departing of minor unreacted byproduct in the precursor solution.
Fluorescence intensity did not show visible change after washing for 5 times, which
indicated the dye molecular has been firmly attached to the silica framework by the
chemical bond.
Table 1 Change of the fluorescence intensity after washing
Washing times |
1 |
2 |
3 |
5 |
7 |
9 |
Fluorescence intensity |
285 |
280 |
277 |
272 |
271 |
271 |
3.3 Photostability experiment
The fluorescein doped silica nanoparticles suspension was irradiated with a deuterium
lamp and then fluorescence was determined to investigate the photostability of the
nanoparticles. The result was compared with AMF solution (Fig.3). No noticeable
photobleaching was observed for ADA-doped nanoparticles in solution for a period of 70min,
the fluorescence intensity decreased to 95% of the initial intensity. However, the
intensity of AMF dropped to 0.9% of the initial intensity. These results suggested that
the ADA-doped nanoparticles are much photostable. It is suggested that the increase of
photostability for ADA-doped nanoparticles is attributed to the following factors: first,
the immobilization of fluorophores in silica network restraints the movement of
fluorophore molecules; second, the silica coating protects against the penetration of
dissolved oxygen into the silica network.
3.4 The effect of pH on fluorescent property
To investigate the effect of surrounding environment on nanoparticles, fluorescence of
the ADA doped nanoparticles were determined in solution of different pH. The result was
compared with monomer precursor ADA (Fig.4). The maximum fluorescence intensity of ADA and
ADA-doped nanoparticles are both at pH 10, the excitation band maxima of ADA is 495nm when
pH >5, while a blue-shift was found when pH<5. However, pH did not show any effect upon shape of the excitation
and emission spectra of ADA-doped nanoparticles and fluorescence intensity of the
nanoparticles only slightly affected by pH. This suggested that the fluorophores doped
inside the nanoparticles are well protected from the surrounding environments. Slightly
variation of the fluorescence intensity may result from the much few dye molecule attached
on surface of the nanoparticles.
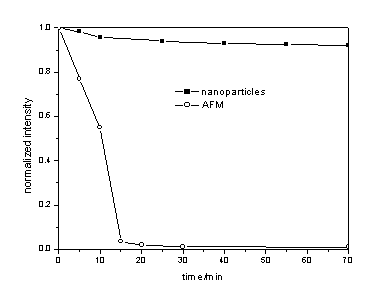
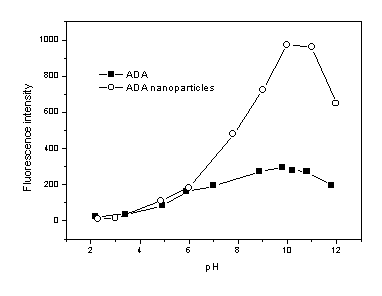
Fig.4 Effect of pH on fluorescence intensity
4 CONCLUSIONS
The present approach of making fluorophore-doped nanoparticles is a general one. There
are several important advantages of these nanoparticles with functionality for
biomolecular label. The nanoparticles are easily prepared using relatively simple
procedures; they have uniform size and good photostability. And most important, this
approach gives a satisfactory result to dye leakage. Surface of the fluorophore-doped
silica nanoparticles can be functionalized in different ways to meet the needs on the
basis of a variety of biomolecular recognition mechanisms. The versatility makes, in our
opinion, ADA-doped nanoparticles a good choice as markers for biochemical assays.
REFFERENCE
[1] Lakowicz J R. Principles of Fluorescence Spectroscopy, second Ed., New York:
Kluwer Academic Publishers/Plenum Publishers, 1999: 531-572.
[2] Trau D, Yang W, Seydack M et al. Anal. Chem., 2002, 74 (21): 5480-5486.
[3] Blaaderen A, Vrij A. Langmuir, 1992, 8 (12): 2921-2931.
[4] Santra S, Zhang P, Wang K M et al. Anal. Chem., 2001, 73 (20): 4988-4993.
[5] Yang W, Zhang C, Qu H et al. Anal. Chim. Acta., 2004, 503 (2): 163-169.
[6] Zhao X, Bagwe R, Tan W. Adv. Mater., 2004, 16 (2): 173-175.
[7] Duan J H, Wang K M, Tan W H et al. Chemical journal of Chinese universities (Gaodeng
Xuexiao Huaxue Xuebao), 2003, 24 (2): 255-259.
[8] Wang X M, Kong W, Xu H et al. Henan chemical industry (Henan Huagong) 1999, 4:14-15.
[9] Ye Z Q, Tan M Q, Wang G L et al. Anal. Chem., 2004, 76(3): 513-518.
宁巧玉, 孟建新*, 王海鸣
(暨南大学化学系,广东 广州 510632)
摘要 采用油包水的反相微乳液法,以5-氨基荧光素衍生而来的发色团为核,成功的制备了一种高效而均一的荧光纳米粒子生物标记物,所制备的纳米粒子具有其独特的优点:易于制备,光稳定性好,且克服了采用传统方法制备核壳荧光纳米颗粒中存在的荧光染料泄漏问题。通过透射电子显微镜表征所得的纳米粒子呈球形,大小均匀,直径在40nm左右,具有生物亲和性,可为生物医学提供一种新型的荧光纳米颗粒标记物。
关键词 5-氨基荧光素, 荧光纳米粒子, 微乳液