| http://www.chemistrymag.org/cji/2005/07c091pe.htm | Dec. 16,
2005 Vol.7 No.12 P.91 Copyright |
Study on the application of aqueous two-phase extraction to purification of some proteins
Li Renqiang1,
Xu Siguang1, Wu Zhongping2, Fang Ling1
(1. Department of Biotechnology, Jinan University, Guangzhou, 510632, China; 2.
Department of Chemistry, Jinan University, Guangzhou, 510632, China)
Received on Sep. 25, 2005; Supported by the Grant-in-Aid for scientific research to Li R from Jinan University, China (640073).
Abstract Based on the advantage of
aqueous two-phase extraction (ATPE) in bioseparation and preliminary experiments that all
heme-containing proteins may be concentrated into the salt phase in aqueous two-phase
system, ATPE was applied to practice for the preparation of these heme-containing protein
Lumbricus terrestris hemoglobin (LtHb), cytochrome c (Cyt c) and peroxidase (POD) from
biomaterials. Results demonstrated that to apply ATPE to extracting process for
preparation of LtHb from earthworm, for preparation of Cyt c from pork heart and for
preparation of POD enzymatic product from radish were successful with the extracting
process being short, operating easily with lower cost. SDS-PAGE, purification effect
analysis and absorption spectral scan explained that very pure LtHb may be obtained, the
extracting process in initial purification of Cyt c was short and effective, and POD
product obtained was almost as good in quality as that by conventional salting out
technique when ATPE were used.
Key words Aqueous two-phase extraction; Purification; Lumbricus terrestris
hemoglobin; Cytochrome c; Peroxidase
Bioseparation is an important technique in
biochemistry, and is also a challenge due to that the bioorganic compounds existed in a
cell lysate is a complicated mixture, including various biomolecules such as nucleic acid,
protein, carbohydrate, lipid, vitamin. Chromatographic methods, electrophoresis,
centrifugation, precipitation are often performed in bioseparation. However, in the case
of unknowing the character of separated compound, it is usually needed at least several
steps to obtain the aim molecules. Bioseparation seems to be complicated.
Aqueous two-phase extraction (ATPE) is a system in that two immiscible
phases are formed when polymers such as poly(ethylene glycol)(PEG) are mixed with dextran,
other polymers, or salts in particular concentration. The compound in ATPE system has an
equilibrium distribution depending on its own surface properties such as charge and
hydrophobicity, and on the physicochemical properties of the two phase, which can be
manipulated by adjusting the factors such as the polymer molecular mass and concentration,
type of phase forming salt, salt concentration, ionic strength, and pH. Recently ATPE has
been widely used for protein separation [1,2] for it has the potential to produce a
concentrated and purified product in one step when compared to the number of steps
involved in conventional bioseparation process. ATPE is a simple separation process, and
it offers gentle nontoxic environments for biomolecules. It is cost effective and can be
scaled up easily, so it is especially suitable for the practical production.
Based on the advantage of ATPE, ATPE composed of PEG and ammonium
sulfate that was often used in practice was applied to the purification of useful
biochemical product or medical protein in this study. According to the preliminary
experiments that the heme-containing proteins such as cytochrome C (Cyt c), hemoglobin
(Hb), peroxidase (POD) could be distributed completely in the phase of ammonium sulfate
salt in PEG―(NH4)2SO4
system, ATPE was used in the process of preparing these biochemical products from some
biomaterials.
1 MATERIALS AND METHODS
1.1 Materials
Earthworm (called Japan red earthworm) with 6-8 cm length, pork heart and radish (Rhaphanus
sativus) were purchased from market. Horse heart Cyt c was from Sigma. All of the
reagents used in the experiments were reagent grade and used as received. Solvent was
distilled water. The instrument for absorption spectral scan was UV-VIS Spectrumlab 54
(Shanghai Lingguang Co., China) and for electrophoresis was applied by Beijing Liuyi
Equipment Factory. Vacuum cold drying apparatus was Heto, High Technology of Scandinavia,
Denmark.
1.2 Preparation of Lumbricus terrestris hemoglobin (LtHb) by ATPE
Washed earthworm body were bleeded in distill water using blender, the supernatant was
obtained after centrifugation of the earthworm homagenate. In ATPE system with 12%(w/w)
PEG6000 and 12%(w/w) ammonium sulfate, the hemoglobin in the supernatant was concentrated
in salt phase. This process eliminated some proteins and enzymes dissolved in PEG phase,
and concentrated the hemoglobin. After the centrifugation for removing the precipitate,
the salt phase was dialyzed against 10mM/L phosphate buffer, pH5.1 overnight, of which the
aim was to precipitate those hemoglobin due to their giant molecular size in near
isoelectric point. This step would separate hemoglobin from other substances. The color
substances were almost precipitated as predicted, and collected using centrifugation,
dried by vacuum cold drying apparatus.
1.3 Application of ATPE to concentration and initial purification of Cyt c from
pork heart
Pork heart with several hundreds grams were bleeded in weak acidic water for the
extraction of Cyt c. The supernatant obtained after centrifugation was added with PEG6000
(12% w/w as final concentration) and (NH4)2SO4 (12% w/w
as final concentration) to make ATPE system, then collected the salt phase in which red
substance were concentrated. After centrifugation, added 20% trichloroacetic acid (TCA)
solution directly to the supernatant with 0.5% TCA as the final concentration to
precipitate proteins, collected the color precipitate using centrifugation. Some water was
added to the precipitate and the solution was dialyzed. The content of Cyt c was measured
using O.D.530 comparing to that of standard horse heart Cyt c.
1.4 Preparation of POD from radish by ATPE and by conventional salting out
Radish of about 300g was bleeded in distilled water and made the extraction for over 1
hr with agitation. Half of the supernatant obtained after centrifugation was used for ATPE
and another half as the control using conventional salting out. PEG (12%, w/w) and (NH4)2SO4
(12% w/w) was made in the supernatant to prepare ATPE system. The salt phase was
dialyzed to remove (NH4)2SO4 and the same volume of cold
acetone was added to precipitate proteins. After centrifugation, cold acetone was again
added to the supernatant according to 0.8∶1,
collected the precipitate that would be POD sample after dialysis. As the control, the
crude enzymatic supernatant was first salting out with 20% saturation of (NH4)2SO4
to remove the precipitate, and again the second salt out with 80% saturation of (NH4)2SO4
to collect the precipitate. After dialysis, the next steps to purify POD using cold
acetone were as the same as that stated in ATPE system.
1.5 SDS-PAGE and visible absorption spectral scan
In SDS-PAGE that could determine the purity of protein, the purified protein or enzyme
as the electrophoresis samples were first mixed with 1%SDS―1% mercaptoethanol solution in a boiled water-bath and then
electrophoresis was performed in 3% concentrate―12%
separation gel.
The visible absorption spectra of LtHb and semifinished Cyt c obtained
by ATPE were examined and compared to that of hemoglobin and Cyt c to convince whether the
aim molecules were obtained.
1.6 Measurement of POD activity
Based on the mechanism that POD may catalyze 2-methoxyphenol to form color compound
with the existence of hydrogen peroxide, POD activity could be examined and presented
using O.D.470. The concentration of POD (protein) could be calculated after
reading O.D.280 and O.D.260 according to the equation:
Concentration of protein (mg/ml) = (1.45 O.D.280-0.74 O.D.260)×diluted fold
So POD activity could be presented as specific activity (O.D.470/min·protein
weight).
2 RESULTS AND ANALYSIS
2.1 SDS-PAGE patterns and purification effect of ATPE
LtHb is a giant molecule with the molecular mass 4-M [3-5], composed of more than 200
chains. Those subunits include four major O2-binding chains, a, b, c, which
form a disulfide-linked trimer, and monomer d. Additional structural chains, "linkers",
are non-heme polypeptide L1, L2, and L3. SDS-PAGE pattern
of LtHb sample obtained by the process described above was shown in Figure 1. Molecular
mass of three bands in the sample was near 22000, 17000 and 15000, respectively, after
comparing to markers. Obviously based on the referent literature [4], the bottom band was
d chain with 15960, and the band near it was the mixture of a, b and c subunit (they were
17525, 16254 and 17289, respectively). The upper band was L subunit that did not contain
heme. At the position near 52000, some weak bands could be seen, which may be explained as
partially disulfide bonds was remained in a, b and c trimer, so a little amount of trimer
remained in SDS-PAGE sample. From Figure 1, LtHb may be convinced to be very pure. Due to
all the color substance was precipitated in ATPE processing, so the recovery of LtHb was
considered as 100%
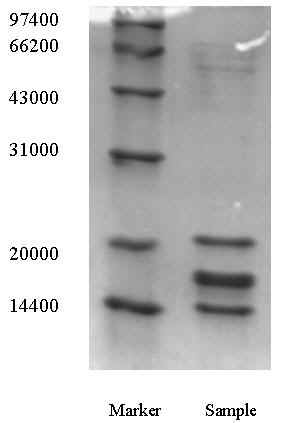
Fig.1 SDS-PAGE pattern of LtHb product
Molecular mass of Cyt c is
12800. Figure 2 showed the electrophoresis result of semifinished Cyt c obtained by ATPE.
The bands in the sample compared to the marker presented that it contained much Cyt c (the
band near 14400 position) although it simultaneously contained some other proteins,
however, Cyt c may be purified easily through a step of weakly acidic cation exchange
column (this was done but not shown here). Purification effect of Cyt c by ATPE was showed
in Table 1, in which the recovery of conventional technique was defined as 100%. The
recovery of Cyt c by ATPE was 87.4, which explained that ATPE had a better purification
effect although it was slightly lower than that of conventional technique, and more
important was that the extraction process for obtaining semifinished Cyt c product using
ATPE was very quick and simple with lower cost, which was suitable for the practical
production. So ATPE was effective in the preparation of Cyt c from pork heart.
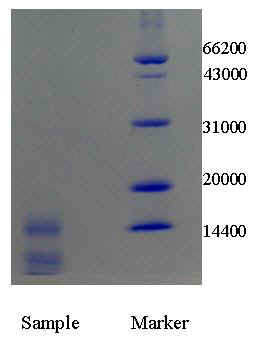
Fig.2 SDS-PAGE pattern of semifinished Cyt c product
Table 1. Comparison of purification effect for Cyt c between ATPE and conventional techniques
Treatment |
Cyt c content of semifinished product (mg/100g) |
Cyt c content of finished product (mg/100g) |
Recovery |
Conventional |
24.56 |
16.84 |
100 |
| ATPE | 21.93 | 14.72 | 87.4 |
In the preparation of POD from radish by ATPE, SDS-PAGE pattern of its product was shown in Figure 3. They were almost the same in purity between conventional salting out and ATPE, which explained that the effect of ATPE was as good as by conventional salting out technique. They all enhanced greatly the purity comparing to the crude enzymatic solution as shown in Table 2. So many bands appeared in samples of ATPE or conventional salting out technique was due to some impurities remained or POD contained isoenzymes, but these did not affect the products as POD enzymatic product. Specific activity of crude enzyme, POD products made by conventional salting out, by ATPE was 0.077, 43.31 and 31.17 (O.D./mg·min), respectively, also demonstrated that ATPE was effective for the preparation of POD from radish due to that its advantage with quick and simple in extracting process and lower cost may remedy a defect in extracting purity. On the other hand, total enzymatic activity in ATPE was larger than that by conventional technique, which explained that the loss of enzyme protein in purification processing by ATPE was lower, and ATPE was suitable for the production on a large scales.
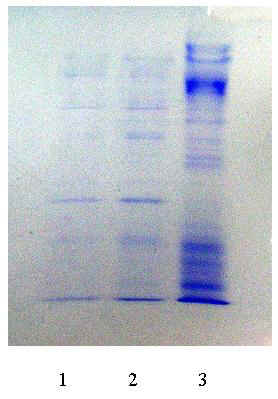
Fig.3 SDS-PAGE pattern of POD product
1:Conventional 2:ATPE 3:Crude enzyme
Table 2 Parameters of POD product made by different methods
|
Crude enzyme |
Conventional salting out |
ATPE |
Total protein (mg) |
132.57 |
2.41 |
4.20 |
| Specific activity (O.D./mg·min) |
0.077 | 43.31 | 31.17 |
| Purification fold | 559.5 | 402.8 | |
| Total activitya
(O.D./min) |
10.2 | 104.3 | 130.8 |
| Protein content (%) | 0.0663 | 0.0012 | 0.0021 |
a
Total enzymatic activity = Specific activity × Total protein2.2. Spectral scan analysis
In order to examine the character of LtHb, Cyt c product obtained by ATPE process,
absorption spectra of these products were measured, which were shown in Figure 4
and Figure 5. These absorption spectra were as the same as those of pure LtHb or pure Cyt
c (data not shown) in both oxide and reduced forms, which explained that the LtHb and Cyt
c obtained by using ATPE remained normal characters.
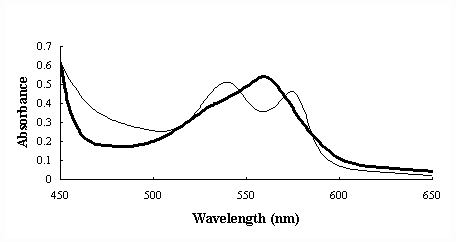
Fig.4 Absorption spectra of Lumbyicus terrestris
hemoglobin product in 450-650nm.
Solid line refers to oxide form and bold solid one to reduced form (adding small
quantities of sodium dithionite to the samples to make reduced form)
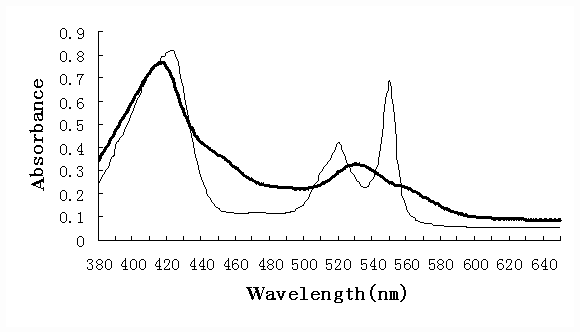
Fig.5 Absorption spectra of semifinished Cyt c sample
Bold solid line refers to oxide form and solid one to reduced form (adding small
quantities of sodium dithionite to the samples to make reduced form)
3 DISCUSSION
Based on the advantage of ATPE and preliminary experiments that all heme-containing
proteins may be concentrated into the salt phase in aqueous two-phase system, ATPE was
applied to practice for the preparation of these heme-containing proteins. Experimental
results demonstrated that ATPE was very effective in extracting process for preparation of
these valuable biochemical products, and the advantage of ATPE was very obvious in
bioseparation. In fact, aqueous two-phase system may be composed of various compounds as
we know, however, PEG and ammonium sulfate were used in this extracting process. This was
at first that ammonium sulfate was good for protecting protein from molecular destruction
at normal room temperature and secondly it was nontoxic and cheap, which made the process
more economical. The composition of aqueous two-phase system should change as needed
according to the character of purified aim substance due to that the aim substance has
different equilibrium distribution in various aqueous two-phase systems. Many compounds,
not only biomacromolecule but also inorganic compound or organic molecule with lower
molecular mass may be separated and concentrated by ATPE. So ATPE is widely applied in
compound separation. Composition of aqueous two-phase system deserved studied for
different aim substance, which presents that ATPE is useful in chemical separation.
Another factor to be considered in ATPE would be the concentration of
composed substance used. The formation of aqueous two-phase system needed enough
concentration of PEG or ammonium sulfate, phase diagram may be got through experiment,
which has two-phase and one-phase regions. Concentrations of PEG and ammonium sulfate must
be selected in two-phase region, and the formation of aqueous two-phase system is quicker
as the concentration of PEG or ammonium sulfate increases, which would shorten the
extracting process, but enhance the cost, especially the equilibrium distribution of some
biomacromolecule are depending on the concentration of composed compounds. So the used
concentration of composed substance should be determined based on experiment where whether
the extracting effect is good. This problem was not existed in this study due to
heme-containing proteins were all distributed in salt phase.
4. CONCLUSION
To apply ATPE to extracting process for the preparation of LtHb from earthworm, for
the preparation of Cyt c from pork heart and for the preparation of POD enzymatic product
from radish are successful. In general, the extracting process becomes short, operating
easily with lower cost when ATPE are used. In the preparation of LtHb from earthworm using
ATPE, very pure LtHb product may be obtained. In the application of ATPE to concentration
and initial purification of Cyt c from pork heart, the extracting process is short and
effective. For the preparation of POD product, ATPE is almost good in quality as
conventional salting out technique.
REFERENCES
[1] Balasubramaniam D, Wilkinson C, Cott K V et al. Journal of Chromatography A, 2003.
989:119-129.
[2] Li Y, Beitle R R. Biotechnol. Prog. 2002,18:1054-1059.
[3] Ownby D W, Zhu H, Schneider K, Beavis R C, Chait B T et al. J. Biol. Chem. 1993,
June, 268: 13539-13547.
[4] Zhu H, Hargrove M, Xie Q, Nozaki Y et al. J. Biol. Chem. 1996 Nov, 271: 29999-30006.
[5] Zhu H, Ownby D W, Riggs C K, Nolasco N J et al. J. Biol. Chem. 1996 Nov, 271:
30007-30021.
双水相萃取法应用于一些蛋白质的提取纯化的研究
李任强1,徐思光1,吴忠平2,方玲1
(暨南大学生物工程学系,广州 510632;暨南大学化学系,广州 510632)
摘要 根据预试中发现所有含血红素蛋白质在双水相萃取中都浓缩于盐相和双水相萃取法在生化分离上的优点,把双水相萃取法应用于从生物材料中提取纯化蚯蚓血红蛋白、细胞色素C和过氧化物酶等含血红素的蛋白质。结果表明,在从蚯蚓中提取纯化蚯蚓血红蛋白,从猪心中提取纯化细胞色素C和从白萝卜中制备过氧化物酶制剂过程中应用双水相萃取法都相当成功,萃取时间短,成本低,容易操作。聚丙烯酰胺凝胶电泳、纯化效果比较及吸收光谱扫描等分析结果说明在这些提取纯化过程中应用双水相萃取可获得较纯的蚯蚓血红蛋白,细胞色素C的粗提时间短而有效,所得的过氧化物酶制剂在质量上与传统盐析法所得的几乎一样。
关键词 双水相萃取,纯化,蚯蚓血红蛋白,细胞色素C,过氧化物酶