http://www.chemistrymag.org/cji/2006/081001pe.htm |
Jan. 10, 2006 Vol.8 No.1 P.1 Copyright |
Hu Peng, Wang Fengxin, Wu Xiumei,
Ouyang Jianming
(Institute of Biomineralization and Lithiasis Research, Jinan University, Guangzhou
510632)
Abstract The influence of
sulfated polysaccharide (LPS) isolated from marine algae Laminarin on the
morphology and phase compositions of urinary crystal calcium oxalate (CaOxa) was
investigated by means of scanning electron microscopy and X-ray diffraction. LPS can
inhibit the growth of CaOxa crystal, prevent the aggregation of calcium oxalate
monohydrate (COM), and induce the formation of calcium oxalate dihydrate (COD). All the
three changes can inhibit the formation of CaOxa stones. This result indicated that LPS
may be a potential inhibitor to CaOxa urinary stones.
Keywords calcium oxalate; urinary stone; algae Laminarin
1. INTRODUCTION
Urolithiasis constitutes a serious health problem that affects a significant section
of mankind. For example, about 10% of the U.S. population suffer from this illness[1].
A survey in Shenzhen city, the most southern city in China, showed the incidence of renal
calculus was 4.87%, being 6.12% in the males and 4.07% in the females[2].
Calcium oxalate (CaOxa) is a major inorganic component of kidney stones. Though in vitro
methods it should reproduce some of the stages of a real biological process, and a number
of researchers are tackling the problem from different directions, the mechanism of the
formation of the stones is not yet clearly understood and a number of questions about the
promoting and inhibiting factors still remain unanswered[3,4].
Sulfated polysaccharides are widespread in nature, occurring in marine
algae and in a great variety of other organisms. In marine algae, they are present as
sulfate fucose (fucoidans) and as sulfate galactans (carrageenans and agars). Recently,
there has been an increasing interest in systematic screening of biological activity of
sulfated polysaccharides isolated from marine algae such as the antitumor, antivirus,
anticoagulant and antihyperlipidemia activities[5,6]. However, there are few
report about the application of seaweed polysaccharides in the inhibition of urinary
stones. Actually, in urine, there are many polysaccharide especially the
glycosaminoglycans (GAGs), including chondroitin sulfate A (C4S), chondroitin
sulfate C (C6S), heparin, hyaluronic acid, and dermatan sulfate, etc[7].
Most of these GAGs have similar molecular structure as LPS and can inhibit the growth of
urinary stones[7-10]. With this in mind, the inhibitive action of LPS on the
crystallization of urinary crystal CaOxa was investigated in this work.
2. MATERIALS AND METHODS
2.1 Reagents
All of the solutions were prepared with reagent-grade chemicals that were purchased
from Shanghai Chemicals Co. Doubly distilled water was used.
2.2. Polysaccharide extraction and fraction[5,6,11]
Marine algae Laminarin was collected on the seaside of Southern Chinese Sea,
Guangdong. It was cleaned with seawater, air dried, stored in dark place at room
temperature and ready for use.
The extraction of polysaccharide from Laminarin was carried out
according to the literature[5,6,11]. The average molecular weight of LPS was
110 000. This LPS consists of linear, mannitol- or glucose-ended chains of b -(1® 3) linked glucose residues, with occasional b -(1® 6)-linked branches. The content of sulfated group in the sample was
25%, which was determined by gelatin-BaCl2 method.
UV-visible spectrum of LPS shows the characteristic peak of LPS at 193
nm. The fact that no peak appeared at 260-280 nm indicated that there is no protein and no
nucleic acid in LPS.
2.3 Crystallization of calcium oxalate
The crystallization experiments of CaOxa were carried out at
temperature 37±1ºC in a constant temperature
chamber. The concentration of both Ca2+ and Oxa2- in metastable
solutions equal 0.30 mmol/L. The concentration of LPS investigated in this work is 0,
0.01, 0.10, 0.40, and 1.0 mg/mL.
2.4 Measurement of calcium oxalate crystals
The morphology of CaOxa was measured by scanning electron microscopy (Phlips XL-30
ESEM) at an operating voltage of 10 KV. X-ray diffraction (XRD) results were recorded on a
D/max-g A X-ray diffractometer (Rigaku, Japan) using Ni-filtered Cu-Ka radiation (l =1.54Å) and a scanning rate of 2° min-1 at 40
KV, 30 mA.
The phase composition of the CaOxa crystals was estimated from the
intensity ratio of the major X-ray diffraction lines of COM (ICOM)
and COD (ICOD), respectively, as shown in eq. (1)[3,12] for
COD, for example:
Estimated ![]() (1)
(1)
with ICOM and ICOD being the intensities of the major
diffraction lines of COM and COD, respectively.
3. RESULTS AND DISCUSSION
3.1 LPS inhibit growth of CaOxa crystals
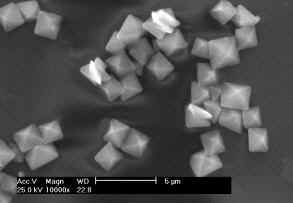
Fig. 1 shows the SEM images of CaOxa crystals grown in the presence of various
concentrations of LPS. It can also be seen that the size of both CaOxa crystals decreased
with the increase of the concentration of LPS. It was closely linked to the complexation
between Ca2+ ions and the negatively-charged sulfate groups of LPS. The content
of sulfate groups in LPS is 25%. These sulfate groups can form the complex with Ca2+
ions in solution. It not only decreases the supersaturation of CaOxa, but also closes the
active sites for the growth of CaOxa crystals, thus making the size of the COM crystals
being small

(a)
(b)

(c)
(d)
(e)
Fig. 1 Scanning electron microscopy of calcium oxalate crystals
grown in the presence of 0 (a), 0.01 (b), 0.10 (c), 0.40 (d), and 1.0 mg/mL (e) of
polysaccharide LPS respectively. The bar=5
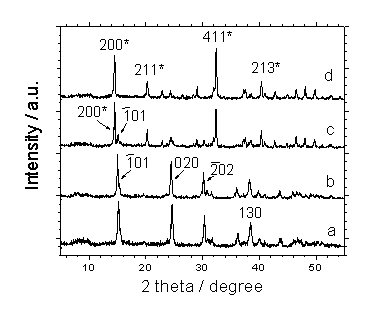
Fig. 2 XRD patterns of CaOxa crystals grown in the presence of 0 (a), 0.01 (b), 0.10 (c), and 1.0 mg/mL (d) of polysaccharide LPS respectively. The diffractive peaks with asterisks show COD and those without asterisks show COM.
3.2 LPS inhibit aggregation of of
calcium oxalate monohydrate (COM) crystals
It can be seen from Fig. 1 that the morphology of the CaOxa crystals was affected by
the concentration of LPS. In the absence of LPS, most of the CaOxa crystals are aggregated
(Fig. 1a). XRD showed that these aggregates crystals were calcium oxalate monohydrate
(COM) crystals. In XRD pattern (Fig. 2a), COM crystals show the diffraction peaks at
0.593, 0.365, 0.298, and 0.236 nm, which can be assigned to the (![]() ), (020), (
), (020), (![]() ), and (130) planes of COM[3,12].
), and (130) planes of COM[3,12].
However, the percentage of the aggregates decreased in the presence of
LPS. It indicated that LPS prevents the aggregation of COM crystals.
3.3 LPS induce the formation of calcium oxalate dihydrate (COD) crystals
It can be seen from Fig. 1 that LPS can induce the formation of bipyramidal COD
crystals. Especially in the presence of 0.40 (Fig. 1d) and 1.0 mg/mL (Fig. 1e) of LPS, the
bipyramidal COD crystals were the dominant phase. In the XRD patterns, the diffraction
peaks at 0.618, 0.442, 0.278, and 0.224 nm were assigned to the (200), (211), (411), and
(213) crystal planes of COD crystals, respectively (Fig. 2c,2d).
Quantitative analyses indicated that in the presence of 0.01, 0.10,
0.40 and 1.0 mg/mL LPS, the percentages of COD are 5, 15, 70 and 95%, respectively. That
is, in a high concentration range from about 0.4 to 1.0 mg/mL, LPS mainly induce the
formation of COD crystals.
There are many evidences that the nucleation and growth of COM in urine
and subsequent trapping of the crystal within the kidney lead to stone formation, while
forming COD crystals may be protective. That is, COM exhibits a greater degree of
attachment to renal tubule cells in culture compared with COD[13]. A
theoretical calculation also indicated that COM has a stronger affinity for these cell
membranes than COD [14]. So it can be concluded that COD is more easily
excreted out of the body than COM. As a result, the induction of more COD crystals will be
in favor of preventing the formation of urinary stone. Therefore, LPS may have the
potential to inhibit CaOxa crystallization directly and may be useful in stone therapy.
The crystallization of CaOxa in the presence of sulfated polysaccharide (LPS) isolated from marine algae Laminarin (LPS) was investigated. LPS can decrease the size of COM and COD crystals, prevent of COM from the aggregation, and increase the content of COD. All the three changes can inhibit the formation of CaOxa stones. This result indicated that LPS may be used as a potential inhibitor to CaOxa urinary stones. Acknowledgments
This research work was granted by the Natural Science Foundation of China (20471024) and the Project Sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education. We thank Prof. Cen Y Z. (Department of Chemistry, Jinan University) for his supply of LPS.
REFERENCES
[1] Sheng X X, Jung T S, Wesson J A, Ward M D. PNAS, 2005, 102 (2): 267.
[2] Xu S H, Chen J Q, Zhou H. Chin. J. Urol. (in chinese), 1999, 20 (11): 655.
[3] Ouyang J M, Duan L, Tieke B. Langmuir, 2003, 19 (21): 8980.
[4] Ouyang J M, Deng S P. Dalton Transactions, 2003, 63 (14): 2846.
[5] Lépagnol Descamps V, Richard C, Potin M, Yvin J C, Kloareg B. Carbohydrate Res, 1998, 310: 283.
[6] Pang Z, Otaka K, Maoka T, Hidaka K, Ishijima S, Oda M, Ohnishi M. Biosci Biotechnol Biochem, 2005, 69 (3): 553.
[7] Ouyang J M, Zhong J-P, Su Z X, Kuang L, Liang W B. Chinese J Urol, 2003, 24: 138.
[8] Ouyang J M, Chen D Z, Zhong J P. Mol. Cryst. Liq. Cryst., 2004, 420: 91.
[9] Ouyang J M, Deng S P, Zhong J P, Tieke B, Yu S-H. J Cryst Growth, 2004, 270: 646.
[10] Iida S, Ishimatsu M, Chikama S, et al. Urol Res, 2003, 31 (3): 198.
[11] Miao H Q, Elkin M, Aingorn E, Ishai-Michaeli R, Stein C, Vlodavsky I. Int J Cancer, 1999, 83: 424.
[12]Ouyang J M, Deng F, Duan L. Coll Surf A, 2005, 257: 215.
[13] Nenow D, Vitkov L. J Cryst Growth, 1997, 182: 461.
[14] Mandel N. J Am Soc Nephrol, 1994, 5: S37.
昆布藻硫酸多糖抑制草酸钙结石的形成
胡鹏, 王凤新, 吴秀梅, 欧阳建明
(暨南大学生物矿化与结石病防治研究所, 广州 510632)
摘要 用扫描电子显微镜(SEM)和X射线衍射(XRD)研究了从昆布藻中提取的硫酸多糖(LPS)对尿结石的主要成分草酸钙晶体(CaOxa)形貌和晶相的影响。LPS不仅能抑制CaOxa晶体的生长,抑制一水草酸钙(COM)的聚集,而且诱导二水草酸钙(COD)形成,这3种变化均有利于其抑制草酸钙结石的形成。本结果表明,LPS是一种潜在的抗草酸钙结石药物。
关键词 草酸钙;昆布藻;尿结石