http://www.chemistrymag.org/cji/2006/082014pe.htm |
Feb. 2, 2006 Vol.8 No.2 P.14 Copyright |
Li Weili, Ma Yinhai, Peng
Yongfang, Zhu Liya#, Huang Zhangjie#
(Department of Chemistry, Kunming Teacher'College, Kunming 650031;#Department of
Chemistry, Yunnan University, Kunming, 650091, China)
Received Dec. 14, 2005.
Abstract In this paper, a
new method for the simultaneous determination of palladium, platinum and rhodium ions as
metal-DHNTR chelates was developed using a rapid column high performance liquid
chromatography equipped with on-line enrichment technique. The palladium, platinum and
rhodium ions were pre-column derivatized with DHNTR to form colored chelates. The Pb-DHNTR, Pt-DHNTR and Rh-DHNTR chelates can be absorbed onto the
front of the enrichment column when they were injected into the injector and sent to the
enrichment column [ZORBAX Stable Bound, 4.6×10 mm, 1.8 mm] with a buffer solution of
0.05 mol/L sodium acetate-acetic acid buffer solution (pH 4.0) as mobile phase. After the
enrichment had finished, by switching the six ports switching valve, the retained chelates
were back-flushed by mobile phase and traveling towards the analytical column. These
chelates separation on the analytical column [ZORBAX Stable Bound, 4.6×50 mm, 1.8 mm] was satisfactory
with 63% acetonitrile (containing 0.05 mol/L of pH 4.0 sodium acetate-acetic acid buffer
salt and 0.01 mol/L of tritonX-100) as mobile phase. The detection limits (S/N=3) of
palladium, platinum and rhodium are 1.4 ng/L, 1.8 ng/L and 2.2 ng/L, respectively. This
method was applied to the determination of palladium, platinum and rhodium in water and
urine with good results.
Keywords palladium; platinum; rhodium; 2,4-dihydroxy-1-naphthalthiorhodanine; rapid
column high performance liquid chromatography; on-line enrichment
1 INTRODUCTION
Environmental contamination by the platinum group elements (PGEs)has received more and
more attentions, and the determination of basal concentrations of those metals has a key
role since an increase of their level [1,2].
However, the heterogeneous composition of samples and the low concentration levels of
palladium, platinum and rhodium involved make the direct measurement of analytes really
difficult. Several analytical techniques have been employed with this matrix in recent
years and most of the advantages and drawbacks have been reviewed [3-7]. The
high performance liquid chromatography method has been proved to be a favorable and
reliable technique [8-11]. However, the routine chromatographic methods need a
long separation time (more than 10 min is needed).
In this paper, a new reagent, 2,4-dihydroxy-1-naphthalthiorhodanine
(DHNTR) was firstly synthesized and used as pre-column
derivatization reagents for palladium, platinum and rhodium. A ZORBAX Stable Bound rapid
analysis column (4.6×50 mm, 1.8 mm) was used for
the separation of Pd-DHNTR, Pt-DHNTR and Rh-DHNTR chelates on a high performance liquid
chromatography equipped with on-line enrichment technique. The palladium, platinum and
rhodium were separated completely within 2.0 min in this method. The separation time was
greatly shortened compared to the routine chromatographic methods. This method can be
applied to the determination mg/L (ppb) level of palladium, platinum and rhodium ions in water
and human urine with good results.
2 EXPERIMENTAL
2.1 Apparatus
On line column enrichment system used is shown in Fig-1. This system includes a Waters
quadripump, Waters 515 pump, Waters 996 photodiode array detector, six ports switching
valve, large volume injector (containing 10.0 ml samples) and column. The enrichment
column is ZORBAX Stable Bound pre-column (4.6×10 mm, 1.8 mm) and the analytical column
is ZORBAX Stable Bound rapid column (4.6×50 mm, 1.8 mm). The pH value was determined with a Beckman F-200 pH meter.
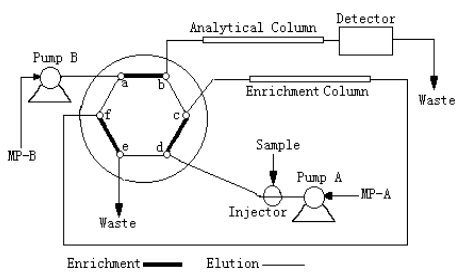
Fig.1 On-line enrichment system using the valve-switching technique
Pump A, Waters 515 Pump. Pump B, Waters 2690 Alliance quadripump. Injector can contain 10
ml of sample. Six ports switching valve (Waters Corporation). Enrichment Column, ZORBAX
(4.6×10 mm, 1.8 m m). Analytical
column, ZORBAX (4.6×50 mm, 1.8 m m).
Detector, Waters 996 photodiode array detector. MP A, 0.05 mol/L of pH 4.0 sodium
acetate-acetic acid buffer solution. MP B, 68% acetonitrile (containing 0.05 mol/L of
pH=4.0 sodium acetate-acetic acid buffer salt and 0.01 mol/L of tritonX-100)
2.2 Synthesis of DHNTR
The DHNTR was synthesized as following procedure: 50 mL of acetic acid was added to
the sample of 1.5 g of thiorhodanine and 1.7 g of 2,4-dihydroxy-1-naphthalaldehyde, and
the mixture was heated gently to dissolve the thiorhodanine and
2,4-dihydroxy-1-naphthalaldehyde completely. The solution was refluxed for about 1.0 h,
and 0.5 mL of concentrated sulfuric acid was added dropwise during refluxing. After the
color of the solution turned red, the refluxing was stopped and the sample was poured into
150 mL of distilled water. To this solution, a small amount of aqueous ammonia was added.
Thereafter, the precipitants were separated by filtration, and were recrystallized twice
with absolute alcohol. The yield is 46%. The structure of DHNTR was verified by elemental
analysis, IR, 1HNMR and MS. Elemental analysis: C14H9NO2S3,
calculated (found), 52.64 (52.47)% C, 2.84 (2.76)% H, 4.39 (4.46)% N, 30.12 (30.05)% S. IR
(KBr) (cm-1): 3600 (n-OH), 3315 (n-N-H);3060, 3020 (n=C-H);1660 (dN-H);1566, 1548,
1515, 1450 (nC=C);1292 (nC-N);1171, 1215 (nC=S);825 (dAr-H);806 (dC=C-H). 1HNMR
(solvent: DMSO-d6) (d,ppm): 4.82 (1H, s, C-OH, H 1); 6.24 (1H, s, Ar-H, H 2); 4.76 (1H,
s, C-OH, H 3);7.54-7.88 (4 H, m, Ar-H, H 4-7); 6.56 (1H, s, -C=C-H, s, H 8). MS (EI)
(m/z): 319 (M+·). All of those experimental results show that the
DHNTR has the following structure, as Scheme 1.
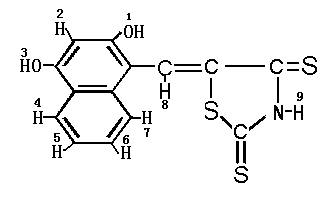
Scheme 1
2.3 Chemicals
All of the solutions were prepared with ultra-pure water obtained from a Milli-Q50 SP
Reagent Water System (Millipore Corporation, USA). Palladium, platinum and rhodium
standard solution: 1.0 mg/ml (Obtained from Chinese Standards Center), a working solution
of 0.2 m g/ml was prepared by diluting this standard solution. HPLC grade acetonitrile
(Fisher Corporation, USA). A sodium acetate-acetic acid buffer solution (0.5 mol/L, pH
4.0) was used. DHNTR solution (2.0×10-4
mol/L) was prepared by dissolving DHNTR with 95% ethanol. Mobile phase A: 0.05 mol/L pH
4.0 sodium acetate-acetic acid buffer solution. Mobile phase B: 63% acetonitrile
(containing 0.05 mol/L of pH 4.0 sodium acetate-acetic acid buffer salt and 0.01 mol/L of
tritonX-100). All other reagents used were of analytical reagent-grade. The glass and
Teflon ware used were soaked in 5% of nitric acid for at least 2 h, and then thoroughly
wash with pure water.
2.4 Standard Procedure
A 0-15 ml of 0.2 m g/ml standard or sample solution was transfered into a 25 ml of
volumetric flask. To which, 4.0 ml of 1.0×10-4 mol/L DHNTR solution, 3 ml of 0.5 mol/L sodium
acetate-acetic acid buffer solution (pH 4.0) and 1.0 ml of 1 % TritonX-100 solution were
added. The solution was diluted to volume with water and mix well. After 10 min, a 10.0 ml
of solution was introduced into injector and sent to enrichment column with mobile phase A
at flow rate of 2.0 ml/min. When the enrichment had finished, by switching the valve of
six ports switching valve, the metal-DHNTR chelates, which absorbed onto the foreside of
enrichment column, were eluted by mobile phase B at the flow rate of 2.0 ml/min in reverse
direction and traveled towards the analytical column. The chelates were separated on the
analytical column. A tridimensional (X axis: retention time, Y axis: wavelength, Z axis:
absorbance) chromatogram was recorded from 400-650 nm with photodiode array detector and
the chromatogram of 545 nm is shown in Fig.2.
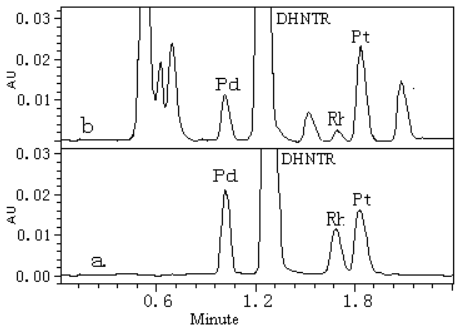
Fig. 2 Chromatogram of standard sample (a)
and Occupationally exposed human urine samples (b). The concentration of palladium and
platinum is 1.0 m g/L in standard sample.
3 RESULT AND DISCUSSION
3.1 Precolumn Derivation
The optimal pH for the DHNTR reacts with metal ions is 2.5 - 5.8 for palladium, 2.8 -
5.2 for platinum, and 2.2 - 4.8 for rhodium, so a 0.5 mol/L of pH 4.0 sodium
acetate-acetic acid buffer solution was recommended to control pH.
It was found that 0.5 ml of 1.0×10-4 mol/L DHNTR solution was
sufficient to complex 5.0 mg
of palladium, platinum and rhodium, respectively. But in real samples, the foreign ions,
such as Hg2+, Pb2+, Cu2+, Ag+ and the like,
form complex with DHNTR and consume reagents. Therefore, the amount of DHNTR must be in excess. In this experiment, 4.0 ml
of 1.0×10-4 mol/L DHNTR solution was recommended.
The experiments show that in the nonionic surfactants or cationic
surfactants medium, the sensitivity of the metal-DHNTR chelates was increased markedly.
Various nonionic surfactants and cationic surfactants enhance the absorbance in the
following sequence: TritonX-100 > Tween-80 > Tween-20 > CTMAB > CPB.
Therefore, TritonX-100 was selected as additive in this experiment. The use of 0.6-1.4 ml
of TritonX-100 solution give a constant and maximum absorbance in this experiment.
Accordingly, 1.0 ml TritonX-100 solution was recommended.
The DHNTR can react with Pd(II), Pt(II) and Rh(III) rapidly. The
reaction was complete for 5 min at room temperature, and the complex was stable for at
least 6 h.
3.2 On-Line Enrichment
Because the Pd-DHNTR, Pt-DHNTR and Rh-DHNTR chelates are stable in weak acid medium.
To avoid the chelates decomposing during the enrichment, a 0.05 mol/L of sodium
acetate-acetic acid buffer solution of pH 4.0 (mobile phase A) was selected as mobile
phase to send the chelates to the enrichment column and a ZORBAX Stable Bound pre-column
(4.6×10 mm, 1.8 m m) with pH range 1-11.5 was
selected as enrichment column. Experiments showed that the volume of 10 ml sample injected
was sensitive enough to determine Pt(II), Pd(II) and Rh(III) in water, urine and soil
samples, so a 10 ml sample injection was recommended.
3.3 Spectrophotometric Properties
The absorption spectrum of metal-DHNTR chelates was obtained by measured with a
Shimidzu UV-2401 spectrophotometer. Results show that the maximum absorption is 545 nm for
Pd-DHNTR chelate, 552 nm for Pt-DHNTR and 542 nm for Rh-DHNTR chelate. Therefore, the 545
nm was selected as detecting wavelength.
3.4 Chromatographic Separation
The experiments showed that the Pd-DHNTR, Pt-DHNTR and Rh-
Pd-DHNTR chelates have a good stability in the presence of weak acid buffer solution and
TritonX-100 medium. The pH of mobile phase within 2.5-3.8 and containing a 0.008-0.12
mol/L of TritonX-100 in the mobile phase can avoid the metal-chelate decomposing in the
course of separation and get a good peak shape. So acetonitrile/water (63/37) (containing
0.05 mol/L of pH 4.0 sodium acetate-acetic acid buffer salt and 0.01 mol/L of tritonX-100)
was selected as mobile phase. To shorten the chromatographic separation time, A ZORBAX
Stable Bound rapid analysis column (4.6×50 mm, 1.8 m m) was selected in this experiment. With rapid
analysis column, the palladium, platinum and rhodium chelates were separated completely
within 2 min. Compared to the routine chromatographic method, more than 85% of separation
time was shortened.
3.5 Calibration Graphs
Under optimum conditions, regression equations of
metal-DHNTR chelates were established based on the standard sample injected and its peak
areas. The limits of detection are calculated by the ratio of signal to noise (S/N=3). The
results were shown in Table 1. The reproducibility of this method was also examined for 10
m g/L of Pd(II), Pt(II) and Rh(III). The relative standard deviations (n=10) were also
shown in Table 1.
Table 1 Regression Equation, Coefficient and Detect limit
Components |
Regression Equation |
Linearity Range |
Coefficient |
Detect limit (ng/L) |
RSD% |
Pd-DHNTR |
A = 2.02×106 C -1589 |
8 - 7500 |
r=0.9992 |
1.4 |
2.8 |
Pt-DHNTR |
A = 1.96×106 C -1422 |
5 - 4600 |
r=0.9987 |
1.8 |
3.2 |
Rh-DHNTR |
A = 1.73×106 C + 1927 |
12 - 6400 |
r=0.9989 |
2.2 |
3.4 |
3.6 Interference
Under the pre-column derivatization
conditions, the foreign ions of Cu(II), Hg(II), Pb(II), Tl(III), Bi(III), Ag(I), Au(III)
can react with DHNTR to form color chelates. To examine the selectivity of this method,
the interference of these foreign ions was investigated.
3.7 Application
Taking an appropriate volume (planting effluents 20 ml, river water 200 ml, human urine 50 mL) of the sample in a 500 mL flask. The samples were concentrated to about 5 mL by heating on a hot plate, and was transferred into a 25 mL teflon high-pressure microwave acid-digestion bomb (Fei Yue Analytical Instrument Factory, Shanghai, China). To which, 2.0 ml of concentrated nitric acid and 3.0 ml of 30% hydrogen peroxide was added. The bombs were sealed tightly and then positioned in the carousel of the microwave oven (Model WL 5001, 1000 W, Fei Yue Analytical Instrument Factory, Shanghai, China). The system was operated at full power for 6.0 min. The digest was evaporated to near dryness. The residue was dissolved with 5 ml of 5% of hydrochloric acid and transferred into a 25 ml of calibrated flask quantitatively, then diluted the solution to the volume with 5% hydrochloric acid. The palladium, platinum and rhodium contents were analyzed by using a proper volume of this solution according to general procedure. The results (deducted the reagents blank) were shown in Table 2.
Table 2 Determination results (ng/g) of the samples
Samples |
Found (ng/g) |
ICP-MS Method (ng/g) |
RSD% (n=5) | Recovery% (n=5) | ||||||||
Pt |
Pd |
Rh |
Pt |
Pd |
Rh |
Pt |
Pd |
Rh |
Pt |
Pd |
Rh |
|
Human Urine |
0.0152 |
0.0212 |
0.0086 |
0.0163 |
0.0205 |
0.0089 |
3.4 |
3.4 |
3.6 |
93 |
91 |
88 |
Human Urine |
0.638 |
0.219 |
0.115 |
0.647 |
0.228 |
0.102 |
3.1 |
2.8 |
3.4 |
89 |
93 |
92 |
Planting effluents |
0.855 |
1.562 |
0.164 |
0.836 |
1.581 |
0.156 |
3.2 |
3.2 |
3.2 |
94 |
93 |
91 |
River water |
0.0512 |
0.0462 |
0.0147 |
0.0523 |
0.0448 |
0.0129 |
3.2 |
3.4 |
3.3 |
88 |
87 |
86 |
4 CONCLUSION
The proposed method has the following
advantages: (1) 2,4-dihydroxy-1-naphthalthiorhodanine
REFERENCE
[1] M E Farago, P Kavanagh, R Blanks et al. Analyst., 1998, 123: 451.
[2] C Wei, G M Morrison. Sci. Total. Environ. 1994, 169:146–147.
[3] M Balcerzac. Analyst. 1997, 122:67.
[4] B Godlewska-Zylkiewicz, M Zaleska. Anal. Chim. Acta. 2002, 462:305.
[5] N Jacubowski, I Feldmann, D Stuewer. Spectrochim. Acta B. 1992, 47:107.
[6] DWollenweber, S Straaburg, G Wunsch. Fresenius J. Anal.Chem. 1999, 364:433.
[7] G Philippeit, J Angerer. J. Chromatogr. B, 2001,760,237.
[8] D Gregurek, C Reimannb, E F Stump. Environ Pollut, 1998,102:221.
[9] R R Barefoot, J C Vanloon. Anal. Chim. Acta. 1996, 364,5.
[10] S Hoshi, K Higashihara, M Suzuki. Talanta. 1997, 44:571.
[11] H Wang, H S Zhang, J K Cheng. Talanta. 1999, 49:1.
李维莉,马银海,彭永芳,朱利亚#,黄章杰#
(昆明师范高等专科学校化学系 云南昆明 650031; #云南大学化学系 云南昆明 650091)
摘要 合成新试剂2,4-二羟基萘-1-亚甲基硫代若丹宁(DHNTR) 作为柱前衍生试剂,在线固相萃取富集,反相液相色谱法测定环境中钯、铂、铑的方法。方法的检测限分别为:钯1.4 ng/L、铂1.8 ng/L、铑2.2 ng/L,该方法用于测定环境样品中低含量的钯、铂、铑,结果令人满意。
关键词 2,4-二羟基萘-1-亚甲基硫代若丹宁(DHNTR),反相液相色谱,钯、铂、铑,在线富集