http://www.chemistrymag.org/cji/2006/084029pe.htm |
Apr. 2, 2006 Vol.8 No.4 P.29 Copyright |
(Department of Chemistry, Shanxi Normal University, Linfen 041004)
Received Jan. 17, 2006; Supported by NSFC (20471034) and Youth Foundation of Shanxi (20051011)
Abstract The topological property and
stability of (BN)n (n=10-35) alternate structures have been
investigated at the B3LYP/6-31G* level of density functional theory. It is found that
stability index of all the isomers obey a simple N4x4 (x = 0, 1, 2) topological
rule characterizing the number of bonds between squares. The relative energies show an
increasing tendency with the rising of N404, and the most stable isomer of (BN)n
has the least number of N4x4 (x = 0, 1, 2). The present study provides a simple
topological criterion that can be used to sort an approximate stability order of all the
isomers and the simple filtering scheme is ideally suited for prescreening the
thermodynamically viable structures of boron nitride clusters.
Keywords Boron nitride, Stability, Topology, nomenclature
1 INTRODUCTION
The discovery of the fullerene C60 by Kroto and R. Smalley[1],
followed by the production of larger fullerenes and carbon nanotubes (CNT's) by
Iijima[2] has given impetus to search for novel fullerene-like boron nitride
structures and open up a new era in materials science and nanoscale engineering. As the
isoelectronic analogues to carbon fullerene, boron nitride nanomaterials with a band-gap
energy of ~6eV and non-magnetism are also expected to show various electronic, optical and
magnetic properties such as Coulomb blockade, photo luminescence, and super paramagnetism[3].
Up to now, extensive studies have been reported on the structure and stability of boron
nitrides (BN)n as nanotubes[4] and cages[5].
Experimentally, Stéphan[6] synthesized the small singer-layer and nested
BN cages under electron irradiation of nanotubes and bulk material. More recently, Oku et
al.[7] synthesized and detected the B24N24 and B28N28
clusters by means of an arc-melting method and laser desorption time-of-flight mass
spectrum. Theoretical computationally, it has been found that the small molecules (BN)x
(x=3-10) favor ring structures
formation while those with x > 10 prefer forming cages[8]. As for the caged
BN structures, there exist two major classes[9]
that exhibit quite different structural features, i.e., fullerene-like structures
consisting of pentagons and hexagons, and alternant structures consisting of squares and
hexagons. Fowler and co-workers[10] suggested that the fullerene-like class is
systematically more stable than the alternant class over the entire range of molecule
size. Conversely, other theoretical studies conclude that alternant class would be
preferred [4d,9], and these findings agree with the more recent study of Strout[]
on B13N13, B14N14, and B16N16.
Interestingly, our previous studies show that the medium-sized structures[12]
comprising octagons also exhibit competing stability. However, the traditional
square-hexagon systems are still more stable for the large cages[13].
By now we know that the stability of carbon fullerenes obeys the
isolated pentagon rule (IPR)[14] and the alternant
boron nitrides is dominated by the isolated square rule (ISR)[15]due
to hetero-fullerenes. Besides, a theoretical study on the even subset[16] of
isomers up to 70 atoms has verified that isolated-pentagon-pair (IPP) BxNx+4
cages are especially stable. Furthermore, it is noteworthy that in fullerene, pentagon is
the main strain factor, and it has been proved that with the increased number of N55
(the number of the shared pentagonal C-C bonds), the relative energies of fullerene C48
increase considerably[17]. As an analogue of fullerene, what would be the
situation of the alternant boron nitride with "steric frustration"?
In this paper, we present a B3LYP/6-31G* density functional theory
study on the structures and energies of all the possible isomers of (BN)n
(n=10-35). It is found that the most stable (BN)n isomer always has all the
least number of N4x4 (x = 0, 1, 2) which topologically characterizing
the bonds between squares. And with increased number of the N404, the
relative energies increase consistently. The result provides useful information to
experimentalists on the formation of other BN clusters.
2 COMPUTATIONAL METHODS
All the structures of the (BN)n (n=10-35) isomers were optimized at the
B3LYP/6-31G* density functional theory, and the corresponding vibrational frequency
calculations were used to characterize the most stable structures to be energy minima
without imaginary frequencies. All neutral cages were treated closed-shell singlet
configurations, and were carried out with the Gaussian 03 program[18].
3 RESULTS AND DISCUSSION
3.1 Structures
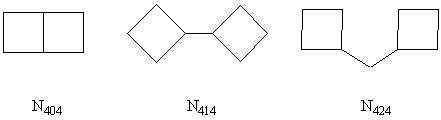
Fig. 1 Structure motifs of the
nomenclature N4x4 (x = 0, 1, 2).
Manolopoulos and his group have suggested a 2D molecular graph of fullerene with faces labeled according to an "orange peel" scheme in 1991[19], i.e., a fullerene structure can be constructed from the order of the pentagonal and hexagonal faces in a continuous face spiral. For example, icosahedral C60 can represent as C60=56666656565656566565656565666665. The scheme can also be applied to (BN)n construction. All the initial structures in this paper were generated based on the fullerene spiral algorithms[20]. Topologically, these neutral cages can be viewed as polyhedra containing squares (f4) and hexagons (f6). In order to get the location of f4 in an easy way, we introduce a N4x4 (x = 0, 1, 2) nomenclature to characterize bonds between f4, in which N404 is the number of shared square B–N bonds among fused f4, N414 is the number of B–N bonds that connected two isolated f4, and N424 is the number of B–N–B or N–B–N linker that bridged two isolated f4 (Fig. 1).
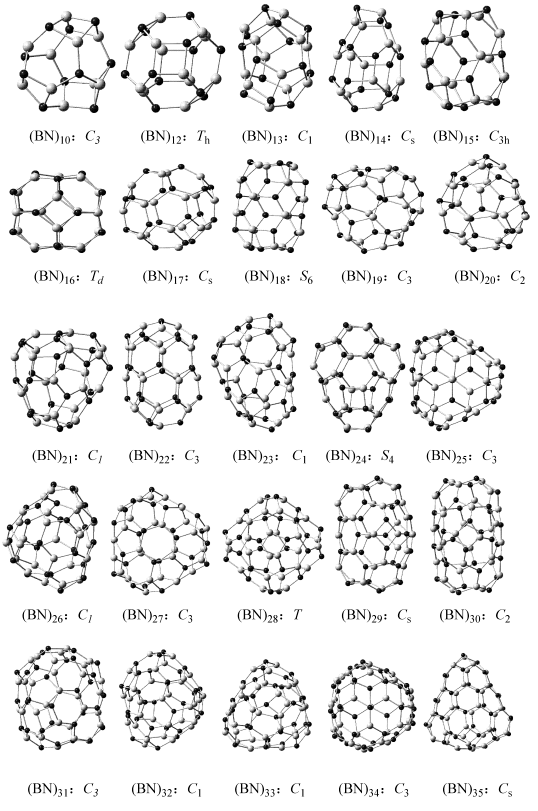
Fig.2 Most Stable (BN)n (n=10-35) isomers
The nomenclature can characterize different
isomers very well, for different isomers at a given size generally have different
nomenclatures. For example, the three isomers of (BN)10 have [3, 3, 6] (a, b, c
in [a, b, c] denotes N404, N414, N424
respectively), [4, 0, 4], [6, 0, 0] nomenclature respectively. Additionally, the
nomenclature can easily describe the f4 adjacencies. For example, the
smallest boron nitride cluster with all f4 isolated, i.e. the isomer
(BN)12: 3 in Th symmetry has nomenclature as [0, 12, 0],
which N404=0 denote the 6 f4 are isolated. Compare
with the all f4 completely isolated nomenclature [0, 0, 0] ( (BN)28:
23, (BN)31: 21, (BN)34: 35 ), (BN)12: 3 still have 12 B–N bonds that bridged f4, and (BN)16: 8
with [0, 0, 24] have 24 B–N–B or N–B–N linkers that bridged two isolated f4.
3.2 Stability
It is the main purpose of this paper to present optimized structures for a large class
of clusters, correlate structural data with relative stabilities and to check the new N4x4
(x = 0, 1 and 2) topological rule. In this situation, a systematic theoretical study would
seem to be valuable if it can provide reliable quantitative estimates for the structures,
relative stabilities. So a systematic calculation of energies and topological properties
has been carried out to all boron nitride alternant structures from (BN)10 to
(BN)35. The total electronic energies and the N4x4 numbers of
all structures are summarized in the supporting Information. The most stable structures of
each given size are shown in Fig. 2. The relative energies of them are defined as
zero.
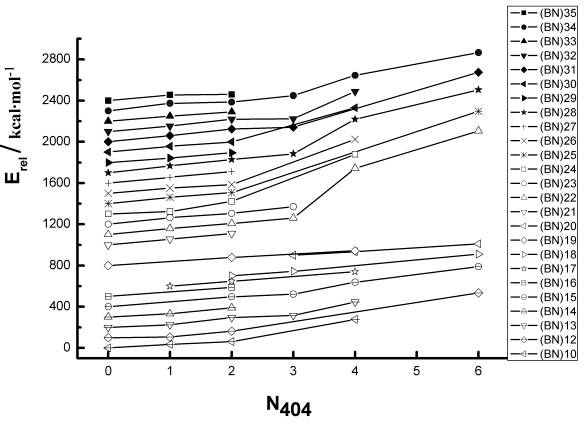
The scale in the vertical coordinate axis is only giving their relative difference at each given size, no relationship between energies of different nuclearity. From the calculation up to 70 atoms in size we carried out, the most striking feature is that, the most stable structure has the minimal value of N404, i.e., the number of shared f4 bonds, while the least stable isomer has the maximum N404 value. The result is well satisfied with the simple rule of thumb ISR [], namely, the most stable structure at any given n is one that minimizes the number of square adjacencies.
The correlations between N404 and relative energies of (BN)n isomers are showed in Fig.3. It is found that with increased number of N404, the relative energies increase steadily and considerably. It shows a general increasing tendency within the rang of (BN)n we tested. Examination of the full set of curves shows that the gradients of (BN)n (n=10-35) are very close, especially for the small cages. For large-sized (BN)n, the curves distort sharply when N404 number over 3. As a whole, the penalty caused by N404 is significant and similar to different sized (BN)n. To be mentioned, it is not claimed that all less value N404 isomers have lower energies than all higher value N404 isomers. As the energy range spanned when the number of isomers grow, some overlaps occur. However, most of them obey this rule and the most stable isomers can be surely included and selected.
Additionally, we studied the other two merits N414 and N424 in detail. For they can provide more detailed information when isomers have the same N404 value. Examination of computed energies of the full set of optimized geometries shows that, the most stable structure of a given size not only has the minimal value of N404, but also preferred to has the minimal N414 number, even N424 number. For example, there are 3 IPR (N404=0) isomers for (BN)22, isomer (BN)22: 14 ([0, 0, 12]) in C3 symmetry that has the minimal N414 number is the most stable, the other isomers (BN)22: 13 ([0, 3, 0]), (BN)22: 12 ([0, 2, 4]) are higher in energy 3.8 kcal/mol, 10.5 kcal/mol respectively. For (BN)28, there are 5 IPR isomers with N414=0, and the most stable one (BN)28: 23 ([0, 0, 0]) in T symmetry has the minimal N424 number.
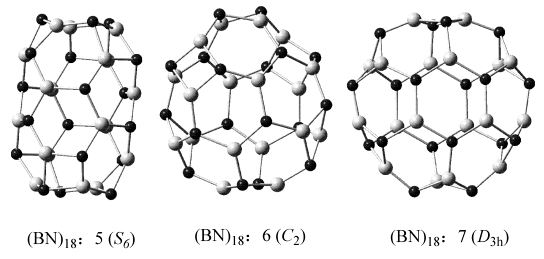
Fig.4 (BN)18 IPR isomers Exceptionally,isomer (BN)18: 6 ([0, 4, 4]) that has the least number of N414 is higher in energy than isomer (BN)18: 5 ([0, 6, 0]) 15.0 kcal/mol, but is lower in energy than another isomer (BN)18: 7 ([0, 6, 0]) 72.2 kcal/mol (Table 1). Fig. 4 gives their structures. We can see that (BN)18: 5 is tube-like, while (BN)18: 6 and (BN)18: 7 are both oblate. As we know, generally, the tube-like isomer is more stable than the oblate one for the strain reason. So (BN)18: 5 that has higher N414 number but tube-like is more stable. As the same reason, isomers (BN)28: 18, (BN)28: 19 that both with [0, 0, 12] nomenclature and C3v symmetry is more stable than (BN)28: 20 with [0, 0, 8] nomenclature that has lower N414 number. We can deem that the stability difference between isomers is also related to the isomer's shape and symmetry. These energetic differences are very similar to those of large sized fullerenes obeying the isolated pentagons rule (IPR) [], and the relative stabilities of them reflect the intimate interplay between strains of individual rings, conjugations and curvatures of the cages. Table 1 B3LYP/6-31G* energies ET (a.u.), Erel relative energies (kcal/mol) and their N4x4
(x = 0, 1, 2) number of some (BN)n isomers
(BN)n |
Sym. |
ET |
Erel |
N404 |
N414 |
N424 |
(BN)18:5 |
S6 |
-1434.4131423 |
0.0 |
0 |
6 |
0 |
(BN)18:6 |
C2 |
-1434.3891857 |
15.0 |
0 |
4 |
4 |
(BN)18:7 |
D3h |
-1434.2742006 |
87.2 |
0 |
6 |
0 |
(BN)26:10 |
C1 |
-2072.1763699 |
0.0 |
0 |
2 |
0 |
(BN)26:12 |
C2h |
-2072.1722159 |
2.6 |
0 |
2 |
0 |
(BN)26:13 |
C1 |
-2072.1671347 |
5.8 |
0 |
1 |
4 |
(BN)26:11 |
Cs |
-2072.1619493 |
9.1 |
0 |
2 |
2 |
(BN)26:9 |
C1 |
-2072.1190854 |
36.0 |
0 |
2 |
4 |
(BN)28:23 |
T |
-2232.1284526 |
0.0 |
0 |
0 |
0 |
(BN)28:21 |
C1 |
-2232.0846379 |
27.5 |
0 |
0 |
4 |
(BN)28:18 |
C3v |
-2232.0633388 |
40.9 |
0 |
0 |
12 |
(BN)28:19 |
C3v |
-2232.0612409 |
42.2 |
0 |
0 |
12 |
(BN)28:17 |
C1 |
-2232.0431705 |
53.5 |
0 |
1 |
4 |
(BN)28:16 |
C1 |
-2232.0361979 |
57.9 |
0 |
2 |
4 |
(BN)28:20 |
C2 |
-2232.0209173 |
67.5 |
0 |
0 |
8 |
(BN)28:22 |
C3 |
-2231.955826 |
108.3 |
0 |
3 |
0 |
(BN)32:19 |
C1 |
-2550.5168243 |
0.0 |
0 |
0 |
4 |
(BN)32:17 |
C1 |
-2550.5155257 |
0.8 |
0 |
1 |
0 |
(BN)32:14 |
Cs |
-2550.5019452 |
9.3 |
0 |
1 |
2 |
(BN)32:18 |
S4 |
-2550.479341 |
23.5 |
0 |
0 |
8 |
(BN)32:15 |
C1 |
-2550.4700165 |
29.4 |
0 |
1 |
2 |
(BN)32:20 |
C1 |
-2550.4514345 |
41.0 |
0 |
1 |
4 |
(BN)32:13 |
C1 |
-2550.4271686 |
56.3 |
0 |
2 |
4 |
(BN)32:21 |
C2h |
-2550.3882612 |
80.7 |
0 |
0 |
8 |
(BN)32:16 |
C2 |
-2550.3848066 |
82.8 |
0 |
2 |
0 |
(BN)35:20 |
Cs |
-2789.6961829 |
0.0 |
0 |
1 |
0 |
(BN)35:15 |
C1 |
-2789.6797162 |
10.3 |
0 |
1 |
0 |
(BN)35:21 |
C1 |
-2789.6735077 |
14.2 |
0 |
0 |
4 |
(BN)35:16 |
C1 |
-2789.6628555 |
20.9 |
0 |
1 |
2 |
(BN)35:14 |
C1 |
-2789.6625762 |
21.1 |
0 |
1 |
2 |
(BN)35:18 |
C1 |
-2789.6141293 |
51.5 |
0 |
1 |
2 |
(BN)35:19 |
C1 |
-2789.6100706 |
54.0 |
0 |
1 |
2 |
(BN)35:17 |
C1 |
-2789.5791583 |
73.4 |
0 |
2 |
0 |
(BN)35:13 |
Cs |
-2789.5552547 |
88.4 |
0 |
2 |
6 |
Based on the discussion above, it can be concluded that, the most stable isomer has the minimal number of N404 while the least stable one has the maximum N404 number, and with increased number of N404, the relative energies increase steadily and considerably. Furthermore, the most stable isomer also preferred to has the minimal N414 number, even N424 number, and the high N424 number may compensate the decrease of energy because of lower N414 number. Comparing the penalty caused by N4x4 (x=0, 1, 2), the deviation between different N404 isomers is very large than those caused by N414 and N424, i.e., N404 is dominant in effecting the relative energies of isomers. Additionally, the stability also relates to the isomer's shape and symmetry. As a filtering principle, the simple N4x4 (x = 0, 1, 2) topological rule characterizing the number of bonds between f4 is very helpful to narrow the range of likely candidates. 4 CONCLUSION
In summary, the topological properties and stabilities of all (BN)n (n=10-35) alternate cages have been investigated at the B3LYP level systemically. It is found that in general, the most stable isomer of (BN)n has the least number of N4x4 (x = 0, 1, 2), and the relative energies show an increasing tendency with N404 number. N404 is always dominant in effecting the relative energies of isomers, but the influences of the N414 and N424 are also significant. The present study suggests a simple topological criterion that can be used to sort an approximate stability order of all the isomers. The simple filtering scheme is ideally suited for prescreening the thermodynamically viable structures of boron nitride clusters. It may also give us a guideline for designing and synthesis of the BN clusters. REFERENCES
[1] Kroto H. W.,Heath J. R., OBrien, S. C., Curl R. F., Smalley R. E. Nature (London), 1985, 318: 162.
[2] Iijima S. Nature, 1991, 354: 56.
[3] Oku T., Kuno M., Kitahara H., Nartia I. Int. J. Inorg. Mater., 2001, 3: 597
[4] (a) Saito Y., Maida M. J. Phys. Chem. A, 1999, 103 (10): 1291 (b) Kongsted J., Osted A., Jensen L., ?strand P.-O., Mikkelsen. K. V. J. Phys. Chem. B, 2001, 105: 10243 (c) Ma R., Bando Y., Sato T., Kurashima K. Chem. Mater., 2001, 13: 2965 (d) Wu H. S., Xu X. H., Zhang F. Q., Jiao H. J. J. Phys. Chem. A, 2003, 107: 6609 (e) Xu H., Ma J., Chen X., Hu Zh., Huo K.-F., Chen Y. J. Phys. Chem. B, 2004, 108: 4024
[5] (a) Alexandre S. S., Nunes R. W., Chacham H. Phys. Rev. B, 2002, 66: 085406 (b) Oku T., Kuno M., Nartia I., Diamond Relat. Mater., 2002, 11: 940 (c) Oku T., Nishiwaki A., Nartia I., Physica B, 2004, 351: 184 (d) Zope R. R., Baruah T., Pederson M. R., Dunlap B. I. Chem. Phys. Lett., 2004, 393: 300
[6] Stéphan O., Bando Y., Loiseau A., Willaime F., Shramchenko N., Tamiya T., Sato T. Appl. Phys. A., 1998, 67: 107
[7] (a) Oku T., Nishiwaki A., Nartia I., Gonda M. Chem. Phys. Lett., 2003, 380: 620 (b) Oku T., Nishiwaki A., Nartia I., Solid State Comm., 2004, 130: 171
[8] (a) Martin J. M. L., El-Yazal J., Fran?ois J.-P., Gijbels R. Chem. Phys. Lett., 1995, 232: 289 (b) Martin J. M. L., El-Yazal J. Chem. Phys. Lett., 1996, 248: 95 (c) Strout D. L. J. Phys. Chem. A, 2001, 105: 261 (d) Jia J. F., Wu H. S., Jiao H. J. Acta Chim. Sin.(Huaxue Xuebao), 2003 , 61(5): 653
[9] (a) Sun M. L., Slanina Z, Lee S. L. Chem. Phys. Lett., 1995, 233: 279 (b) Slanina Z., Sun M. L., Lee S. L. NanoStruct. Mater., 1997, 8: 623
[10] Rogers K. M., Fowler P. W., Seifert G. Chem. Phys. Lett., 2000, 332: 43
[11] Strout D. L. Chem. Phys. Lett., 2004, 383: 95
[12] (a) Wu H. S., Jiao H. J. Chem. Phys. Lett., 2004, 386: 369 (b) Wu H. S., Xu X. H., Jia J. F. Acta Chim. Sin.(Huaxue Xuebao), 2004, 62(1): 28. (c) Cui X. Y., Wu H. S. Chin. J. Chem.( zhongguohuaxue), 2005, 23: 117
[13] (a) Wu H. S., Cui X. Y., Qin X. F., Jiao H. J. J. Mol. Struct.(THEOCHEM), 2004, 714: 153 (b) Wu H. S., Cui X. Y., Xu X. H. J. Mol. Struct. (THEOCHEM), 2005,717: 107
[14] (a) Kroto H. W. Nature (London), 1987, 329: 529. (b) Schmalz T. G., Seitz W. A., Klein D. J., Hite G.E. J. Am. Chem. Soc., 1988,110: 1113.
[15] Fowler P. W., Heine T., Mitchell D., Schmidt R., Seifert G. J. Chem. Soc. Faraday Trans., 1996, 92(12): 2197
[16] Fowler P. W., Rogers K. M., Seifert G., Terrones M., Terrones. H. Chem. Phys. Lett., 1999, 299: 359
[17] Wu H. S., Xu X. H., Jiao H. J. J. Phys. Chem. A. 2004, 108:3813
[18] M. J. Frisch, G. W. Trucks, H. B. Schlegel,et al. Gaussian 03, Revision A.1, Gaussian, Inc., Pittsburgh PA, 2003.
[19] Manolopoulos D. E., May J. C., Down S. E. Chem. Phys. Lett. 1991, 181: 105
[20] Fowler P. W., Manolopoulos D. E., An Atlas of Fullerenes, Oxford: Oxford University Press, 1995
[21] Cioslowski J. Rao N. Moncrieff D. J. Am. Chem. Soc., 2000, 122: 8265
(BN)n笼的稳定性及笼中四元环间键连类型对笼稳定性的影响
武海顺 田欣欣
(山西师范大学化学与材料科学学院 临汾 041004)
摘要 采用密度泛函B3LYP/6-31G*方法对(BN)n (n=10-35) 四六结构的拓扑学性质和稳定性进行了研究。结果发现它们的稳定性顺序遵循一个简单的用来描述四元环间键连类型的拓扑学规则N4x4 (x = 0,1 ,2)。计算表明,各个异构体笼的相对能量随N404值的增大而增大,且最稳定构型具有最小的N4x4 (x = 0, 1,2)值。这一简单的拓扑学标准可用来预测各异构体大概的相对稳定性顺序并能预先挑选出热力学稳定的氮化硼笼状结构。
关键词 氮化硼 稳定性 拓扑学 命名法