http://www.chemistrymag.org/cji/2006/086040pe.htm |
Jun. 1, 2006 Vol.8 No.6 P.40 Copyright |
On-line solid-phase extraction of arsanilic acid from water with arsanilic acid imprinted monolith
Sun Hanwen, Zhao Xiaoli(College of Chemistry and Environmental Science, Hebei University, Key Laboratory of Analytical Science and Technology of Hebei Province, Baoding, 071002, China) Received Apr. 10, 2006; Supported by the National Natural Science Foundation of China (No 203110). Abstract The synthesis and evaluation of a molecularly imprinting polymeric monolith (MIPM) as an on-line selective solid-phase extraction column, for the efficient preconcentration and determination of arsanilic acid (PABAA) in water was reported. MIPM was prepared by in situ polymerization using PABAA as the template molecule, methacrylic acid as the functional monomer and ethyleneglycol dimethacrylate as the cross-linking monomer in acetonitrile and methanol solvent. The recognition mechanism and retention behavior of the MIPM were examined and subjected to a comparison to non-imprinted blank monolithic column (CP, in the absence of template) and 3-NHPAA monolithic column (3-NHPAA-MIPM, template is 3-NHPAA). The retention of PABAA on the MIPM could be reduced by increasing the ratio of water to MeOH: ACN (1:1, v/v) in eluting buffer and this solvent mixture (10ng/L of template) was used for enriching the analyte. The influence of enrichment time and flow rates was investigated and optimized. Using on-line solid-phase extraction MIPM, enrichment time of 5h and flow rates of 0.5mL/min, resulted in a nominal enrichment factor of 83. The off-line solid-phase extraction of batch extraction of PABAA from standard solutions was also investigated and resulted in a considerable enrichment of PABAA but not as nominal as on-line solid-phase extraction.
Keywords Monolithic molecular imprinted; On-line; Solid-phase extraction; Arsanilic acid; Roxarsone 1 INTRODUCTION
Chromatography using molecularly imprinted media has attracted growing attention due to its high selectivity contributed by the matrixes prepared with a chosen target molecule as template[1]. Recently, a great number of studies have been attempted by using a wide range of compounds as print molecules, and this approach appeared particularly effective in the enrichment and purification of small molecules [2-7].
The potential range of applications for MIPs is very extensive. Although the application of MIPs as sorbents in molecularly imprinted SPE (MISPE) was firstly described in 1994 [8], a few studies have been developed [9]. MISPE has been mainly applied in off-line mode to chromatographic systems, with few applications having been developed thus far in on-line mode [10-15]. In two of these on-line methods [11,12], two successive pre-columns packed with C18 -silica and MIP sorbents, respectively were on-line coupled to a liquid chromatographic system to extract selectively a group of triazines from environmental water. On the first pre-column, which contained the C18 -silica, all the compounds were retained. When they were eluted subsequently from this pre-column only the template and the structural related compounds were retained by the second pre-column. In a similar way, Koeber et al. [16] also used two successive pre-columns, but in this case the first one was packed with a restricted access material (RAM) and the second one with a MIP to selectively extract triazines from river water samples. Haginaka and Sanbe [13] used one pre-column, a combined RAM-MIP pre-column, to extract ibuprofen from plasma. The use of only one pre-column as opposed to two in on-line MISPE clearly offers significant advantages in terms of the ease of method development.
Arsanilic acid (p-aminobenzenearsonic acid, PABAA) and roxarsone (3-nitro-4-hydroxyphenylarsonic acid, 3-NHPAA) are an anthropogenic organoarsenic compounds that are used widely as an additive to poultry and hog feed for increased rate of weight gain and improved feed efficiency. The widespread usage of arsanilic acid and roxarsone has led to recent concern of these two compounds. Pentavalent arsenate is an environmental carcinogen. The limit of detection is too high (260mg/L) for effective application to most environmental samples [17].
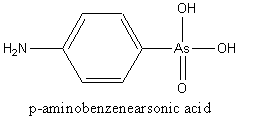
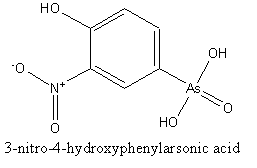
Fig.1 The chemical structures of PABAA (left) and 3-NHPAA
We directed our attention to apply molecular imprinting technology to PABAA enrichment. In the present study, a PABAA imprinted polymeric monolith made by in-situ polymerization was developed. Based on the previous study [18], the polymer was prepared using PABAA as the template, methacrylic acid as the functional monomer and ethyleneglycol dimethacrylate as the cross-linking monomer in acetonitrile and methanol solvent. The application of PABAA imprinted monolith to the enrichment of PABAA from water was attempted and the concentrated PABAA were analyzed by Chromatography.
2. EXPENRIMENTAL2 .1 Materials and apparatus
Arsanilic and roxarsone were donated by RongYao chemical factory (Zhejiang, China). All organic solvents used (methanol (MeOH), acetonitrile (ACN), THF and acetone) were of HPLC grade. Ethylene dimethacrylate (EDMA) was purchased from Fluka. 2,2'-Azobisisobutyronitrile(AIBN) were obtained from Beijing Chemical Reagent company (Beijing, China). Methacrylic acid (MAA) was purchased from Tianjin No.1 Chemical Reagent Factory (Tianjin, China). EDMA and MAA were purified by distillation to remove inhibitor and AIBN was recrystallized prior before use. All of the solutions and samples were filtered through a 0.45um membrane filter (Millipore) before use.
Chromatography was performed with a Shimadzu HPLC system (Shimadzu, Kyoto, Japan). This system is equipped with a photodiode array detector (SPD-M10Avp), a gradient controller (SCL-10Avp), a workstation (CLASS-vp), a degasser (DGU-12A), a column thermostat (CTO-10Avp). A Shimadzu UV-265 UV-VIS spectrophotometer (Shimadzu, Japan) was used to detect UV absorption. A Jintan SYZ-550 high pure water distiller (Jiangsu, China) was used to prepare twice distilled water.
2.2 Preparation of the MIPM and the control monolith
The stationary phase was directly prepared by in-situ polymerization within the confines of a stainless-steel chromatographic column tube of 150×4mm I.D. The template (PABAA 0.5mmol or 3-NHPAA 0.5mmol) and the free-radical initiator (AIBN, 0.125mmol) were dissolved in 2ml of mixtures solvent [ACN-MeOH (3:1 (v/v))], 2mmol of functional monomer (MAA), 20mmol of cross-linker (EDMA) and porogenic solvents (dodecanol (1.5mL) and cyclohexanol (3mL)) were added to the solution. The solution was sonicated for 15 min and purge with nitrogen gas for 10 min at room temperature in order to remove the polymerization inhibitor oxygen. Both sides of the stainless-steel tube were connected with plastics tubes, then sealed at the bottom, filled with the above polymerization mixture. Polymerization was performed by heating the mixture at 50℃ in water bath for 24h. Next the seals and the plastics tube were removed. The column was provided with fittings, and connected to an HPLC pump and washed on-line in turn with THF, methanol/ acetic acid (4:1, v/v) to remove the templates and porogenic solvents. At last, it was washed with MeOH to remove the acid. CP (in the absence of template) and 3-NHPAA-MIPM (template is 3-NHPAA) were prepared and treated in an identical manner.
2.3 HPLC analysis
One of the sample reservoirs was utilized for enrichment solution, PABAA enrichment solution (1.0,10μg/mL) was prepared by dissolving appropriate PABAA in ACN:MeOH:H2O(15:15:70,v/v). The PABAA-MIPM column was washed with PABAA enrichment solution for several hours consecutively and PABAA was concentrated on MIPM column. Then, the column was washed with enrichment solution just without PABAA for 20 minute. At last the desorbing mobile phase was delivered to carry the concentrated PABAA to the UV Detector.
HPLC conditions employed were as follows; mobile phase: ACN:MeOH:H2O(35:35:30,v/v)flow rate for analysis: 1.0 mL/min, flow rate for preconcentration: 0.5 mL/min, UV detection: at 260nm. The total void volume was determined by the injection of acetone.
2.4 Batch extraction of PABAA from veterinary standard solutions
After the chromatographic experiments had been completed, the column was washed with methanol/acetic acid (4:1 v/v) for 1 h. The bottom column fitting was removed and the monolith inside the column was pushed out of the tube using the pressure of the methanol mobile phase at a flow-rate of 5 mL/min. The cylindrical monolith was dried under vacuum at 40℃ for 24 h and then it was ground to pass through a 360-mesh sieve. Prior to their use the particles were washed twice with methanol/acetic acid 7:1 (v/v) in batch mode, followed by two times washing with methanol. In order to re-suspend the fine particles after filtration they were ultra-sonicated after adding the solvents.
The concentration of the individual veterinary solutions were chosen with a value of 1.0 mmol/L, but exactly determined via UV. After that ,100mg each of the PABAA-MIPM 、3-NHPAA-MIPM and CP particles dried under vacuum at 40℃ was weighed into a 10mL concial flask and mixed it with 5.0 mL of 1mmol/L PABAA or 3-NHPAA methanol solution, respectively, shaken on a rocking desk. After a defined period of time, 0.1mL samples were taken from these batches, centrifuged to separate fine particles from the supernatant, followed by the UV determination of the remaining concentration in the supernatants. ACN–MeOH 3:1 (v/v) was also used as solvent for the extraction procedure for comparison. 3. RESULTS AND DISCUSSION
1. On-line MISPE
The effectiveness of the imprinting process can be shown by the retention of the template on the MIPM and the CP. Before use they were washed with MeOH containing 1% (v/v) acetic acid to verify that there was no residual template (PABAA) present.
3-NHPAA has a nitrite group (–NO2) and adjacent group–OH, which can form strong intramolecular hydrogen bond with six-membered ring. Because there were no specific binding sites, enough stable 3-NHPAA–MAA complexes would not be formed in the imprinting process. In spite of their similar structures to 3-NHPAA, PABAA contains hydrophobic aromatic ring and an active amino group. The amino group may form ionic interaction with the ionizable carboxyl group of function monomer MAA. The arsenic acid group of PABAA has its three oxygen atoms, two of which holds dissociable protons. It maybe take part in the interaction process, however, the retention of PABAA on PABAA-MIPM column is considered as mainly based on the contribution of methacrylic acid functionality on the stationary phase. The interaction between the acid group in MIP and the amino group in the template is the most important factor for the recognition of the template molecules by the MIP. The imprinting process is shown in Fig. 2.
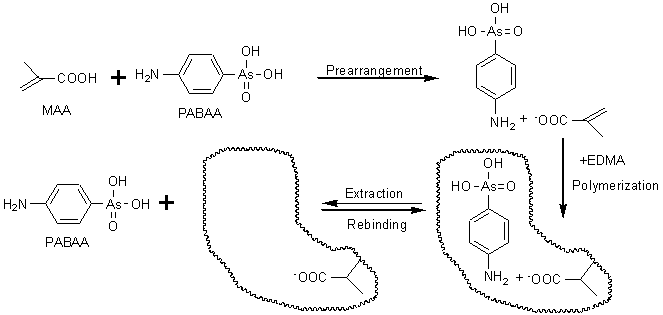
Fig. 2 The imprinting process
Therefore, both Fig.3
(chromatograms for the retention of the PABAA on PABAA-MIPM column and on CP column) and
Table 1, 2 (batch extractions) indicate that PABAA-MIPM has high molecular recognition
ability.
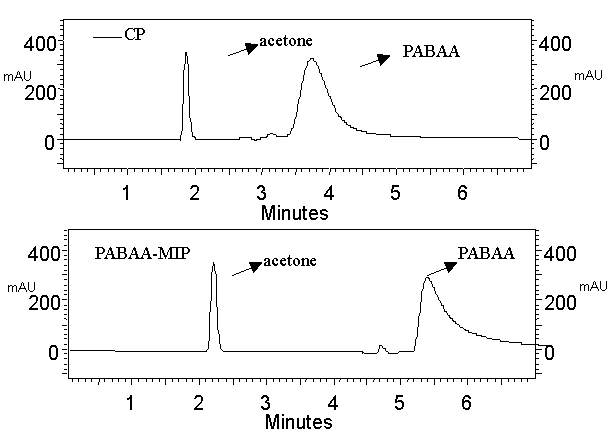
Fig. 3 Analysis of PABAA.
HPLC conditions: column, 4.0mm I.D.×150mm; mobile phase, ACN:MeOH:H2O(35:35:30,v/v)flow rate,
1mL/min; detection wavelength, 260nm; sample composition: 1mg/mL PABAA in mobile
phase/acetone 95:5(v/v); top: CP stationary phase; bottom: PABAA-MIP stationary
phase
The value of the capacity factor
was calculated based on the equation: k'=(tR-t0)/t0
, where tR is the retention time of PABAA or 3-NHPAA, and t0 is the
elution time of the acetone (as a void marker). The separation factors for evaluation of
the selectivity were calculated by determining the ratio of the capacity factors of the
MIPs and the CP using the equations:
aPABAA= k'PABAA-MIPM / k'CP,
In optimizing polymerization conditions , the highest a-value (1.508) of PABAA was found for PABAA-MIPM, along with a value of 0.976 of 3-NHPAA, and a values(1.103) of 3-NHPAA and a values(0.950) of PABAA were found for 3-NHPAA-MIPM.
The elution curves of PABAA on the MIPM with different ratios of water to MeOH:ACN (1:1, v/v) in the mobile phase are shown in Fig. 4, in which the increase of the ratio of water to MeOH:ACN (1:1, v/v) leads to a corresponding reduction of the retention time of PABAA.
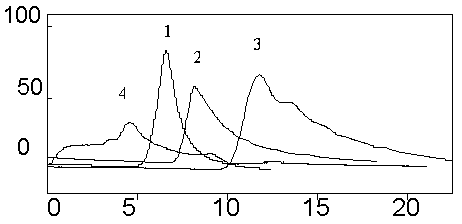
Fig. 4 Elution of PABAA on the MIPM column with different ratios of H2O to MeOH: ACN (1:1,v/v) (1)ACN:MeOH:H2O(35:35:30,v/v)(2)ACN:MeOH:H2O(30:30:40,v/v) (3)ACN:MeOH:H2O(25:25:50,v/v) (4)ACN:MeOH:H2O(15:15:70,v/v)
Conditions: flow rate, 0.5 mL/min; injection volume, 20 mL; concentration, 1 mg/mL in MeOH
It is known that the
template molecule can interact with its molecularly imprinted polymer via hydrogen
bonding, ionic, p-p and/or hydrophobic interactions. Reduction of the retention time of
PABAA as a result of increasing the ratio of water to MeOH:ACN (1:1, v/v) in eluent,
indicated that ionic and hydrophobic were probably the major interactions between MIPM and
PABAA.
There are almost not any PABAA can be eluted with a mobile phase
composition of ACN:MeOH:H2O(15:15:70,v/v)within 60min and even more hours. So we choose ACN:MeOH:H2O(15:15:70,v/v)
contains 10mg/mL of PABAA
as enrichment solution.
The effect of the enrichment solution flow rate through the MIPM was
checked. No significant change in the PABAA was observed for flow rates between 0.25 and
2.0 mL/min. The pump delivered enrichment solution through MIPM for several hours for flow
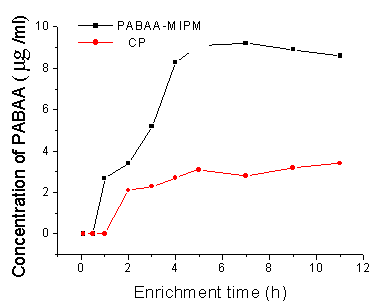
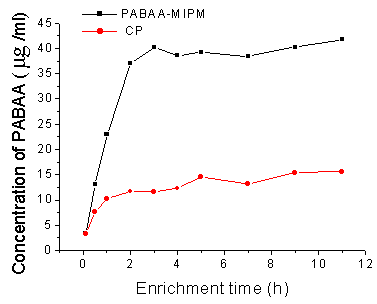
rates 0.5mL/min consecutively. This result shown in Fig.5 indicated that the concentration
of template rise faster in 4h and rise slower after that. Taking into account that longer
enrichment time increased the time of analysis process, 4h was selected for further work.
The result is identical with the batch extraction conclusion and can be explained by the
fact that the column gets a possible saturation of the MIP capacity. For 4h enrichment,
the process resulted in a nominal enrichment factor of 83 for PABAA. The enrichment factor
was calculated by comparing the amount of PABAA detected with the UV response from a
standard solution calibration curves.
(a)
(b)
Fig. 5 Influence of enrichment time on the rebinding/extraction efficiency
for PABAA using PABAA-MIPM and CP. PABAA standard solution:1.0μg/mL(a),10μg/mL(b), enrichment
flow rate,0.5 mL/min. mobile phase, ACN:MeOH:H2O(35:35:30,v/v)flow
rate,1mL/min
Only one specific combination led to an expected specific adsorption on its respective MIPs. When using the different MIPs for the batch extraction of their individual from standard solutions in MeOH and ACN-MeOH 3:1(v/v) solution, the PABAA-MIPM was able to extract more PABAA from that solution than the CP, but for the 3-NHPAA-MIPM unspecific behavior was observed in comparison with the CP. Table.1 and Table.2 shows the results for the extractions from methanol and ACN–MeOH 3:1 (v/v) solution, respectively .Comparable to the MeOH based extractions, the PABAA-MIPM adsorbed more PABAA from an ACN-MeOH 3:1(v/v) solution than the CP, although the difference was not that pronounced. The MIPM showed a higher affinity than the CP which might be explained by the fact that the ACN–MeOH 3:1 (v/v) solution was also used as solvent and swelling effects of the polymer were similar.
In some cases, the supernatants showed even higher concentrations of the PABAA and 3-NHPAA than the original solution which was added to the polymer. This was not only for the MIPs which could have been explained by bleeding of template from the imprints, but also for the CP which had not been in contact to the template/analyte prior to the extraction. Most probably this is due to a certain lack of sensitivity of the analytical method which furthermore, could have been disturbed by, e.g., remains of monomers bleeding from the polymers resulting in additional UV-absorption.
Table 1.Results for the batch extractions of veterinary from methanol solutions using the respective MIPM or CP fine particles
Extraction time(h) |
Original solution |
CP |
PABAA-MIPM supernatant |
3-NHPAA-MIPM supernatant |
|
PABAA |
5 |
0.913 |
0.888 |
0.759 |
0.801 |
27 |
0.883 |
0.791 |
0.724 |
0.792 |
|
98 |
0.920 |
0.741 |
0.665 |
0.702 |
|
3-NHPAA |
5 |
1.034 |
1.087 |
1.125 |
1.149 |
27 |
0.998 |
1.032 |
1.036 |
1.025 |
|
98 |
0.979 |
0.910 |
1.011 |
0.838 |
|
Table 2.Results for the batch extractions of veterinary from ACN-MeOH 3:1 (v/v) solutions using the respective MIPM or CP fine particles
Extraction time(h) |
Original solution ( mmol/L) |
CP |
PABAA-MIPM supernatant |
3-NHPAA-MIPM supernatant |
|
PABAA |
5 |
0.985 |
0.910 |
0.772 |
0.874 |
27 |
0.972 |
0.820 |
0.532 |
0.772 |
|
98 |
0.915 |
0.841 |
0.358 |
0.739 |
|
3-NHPAA |
5 |
0.886 |
0.973 |
1.065 |
1.038 |
27 |
0.910 |
0.918 |
0.905 |
0.863 |
|
98 |
0.854 |
0.788 |
0.746 |
0.714 |
|
The present study started with in situ synthesis of PABAA imprinted monolith (PABAA-MIPM). Then, the recognition mechanism and retention behavior of the MIPM were examined and subjected to a comparison to CP and 3-NHPAA-MIPM.The results described above demonstrated the practicality of the on-line coupling of MISPE to liquid chromatography and also showed fairly well that a correct design of molecularly imprinted polymers for veterinary such as PABAA, allowing a selective and effective preconcentration of the target analyte. This successful preconcentration resulted in a nominal enrichment factor of 83 for PABAA in 4h with flow rates of 0.5mL/min.
Although quite a lot of experimental variables can affect the rebinding/extraction process, once these are optimized, the use of MIPs provides important practical advantages is comparison with other solid-phase extraction methodologies. In particular, the methodology developed in this work demonstrates to be useful for the selective determination of PABAA in water at a low concentration level. REFERENCES
[1] Zhang M L, Xie J P, Zhou Q et al. J Chromatogr. A.,2003,984 :173.
[2] Caro E, Masqué N, Marcé R M, et al. J. Chromatogr. A.,2002,963:169.
[3] Venn R F, Goody R J, Chromatographia.,1999,50:407.
[4] Xie J C, Zhu L L, Luo H P, Zhou L, Li C X, Xu X J, J.Chromatogr.A ., 2001,934:1.
[5] Bjarnason B, Chimuka L, Ramstrom O, Anal. Chem., 1999,71: 2152.
[6] Ferrer I, Lanza F, Tolokan A, Horvath V, et al, Anal. Chem.,2000,72:3934.
[7] Koeber R, Fleischer C, Lanza F, et al, Anal. Chem.,2001,73 :2437.
[8] Sellergren B, Anal. Chem., 1994,66:1578.
[9] Masqué N, Marcé R M, Borrull F, Trends Anal. Chem.,2001,20:477.
[10] Masqué N, Marcé R M, Borrull F,et al, Anal. Chem., 2000,72: 4122.
[11] Ferrer I, Barcelo D, Trends Anal. Chem., 1999,18:180.
[12] Bjarnason B, Chimuka L, Ramstr?m O, Anal. Chem., 1999,71:2152.
[13] Haginaka J, Sanbe H, Anal. Chem., 2000,72:5206.
[14] Guzmán-Vázquez de Prada A, Martínez-Ruiz P, Reviejo A J, Anal. Chim. Acta .,2005,539:125.
[15] Mena M L, Martínez-Ruiz P, Reviejo A J, et al.,Anal.Chim.Acta., 2002,451:297.
[16] Koeber R, Fleischer C, Lanza F, et al,Anal.Chem., 2001,73:2437.
[17] Roerdink Aaron R, Aldstadt III Joseph H,J. Chromatogr. A., 2004,1057:177.
[18] Sun H W,Zhao X L,Liu Z F,et al. Chemistry:An Indian journal.,2005,2(8).
分子印迹整体柱对水中痕量阿散酸的在线富集
孙汉文, 赵晓丽
(河北大学化学与环境科学学院,河北省分析科学与技术重点实验室,河北保定,071002)
摘要 采用分子印迹原位聚合技术合成了对阿散酸具有专属识别能力的分子印迹聚合物整体柱(molecularly imprinting polymeric monolith ,MIPM),建立水中痕量阿散酸的在线预富集及检测方法。以阿散酸为模板分子,以甲基丙烯酸(MAA)为功能单体,乙二醇二甲基丙烯酸酯( EDMA) 为交联剂,采用原位聚合法在MeOH: ACN (1:3, v/v)极性溶液里制备了分子印迹整体柱。通过与洛克沙生印迹柱和非模板分子印迹柱保留能力的比较,讨论了分子识别机理。选择阿散酸浓度为10ng/l的ACN:MeOH:H2O(15:15:70,v/v)混合溶液作为富集条件,考察了富集时间及流速对富集倍数的影响。利用制得的在线固相萃取MIPM,流速0.5mL/min,富集5h,富集倍数为83.对离线固相萃取结果与在线固相萃取结果进行了比较。该方法实现了水介质中阿散酸分子的专属的灵敏的检测。
关键词 分子印迹整体柱,在线,固相萃取,阿散酸,洛克沙生