http://www.chemistrymag.org/cji/2006/087046pe.htm |
Jul. 1, 2006 Vol.8 No.7 P.46 Copyright |
(1 State Key Laboratory of Heavy Oil Processing, China University of Petroleum, Beijing 102249; 2Department of Chemistry, Hebei University of Science and Technology, Shijiazhuang, 050018, China)
Received Jan.18, 2006.
Abstract Thiophene(C4H4S)
are typical thiophenenic sulfur compounds that existed in flow catalytic cracking (FCC)
gasoline. Oxidation reactions of C4H4S were conducted with hydrogen
peroxide (H2O2) and formic acid over a series of metal-loaded
alumina. The effects of loaded metals, temperature, solvent and phase transfer catalyst on
sulfur removal were investigated in detail. The results showed that the copper-loaded
alumina was very active catalyst for oxidation of C4H4S in H2O2/
formic acid system. The oxidation of C4H4S was performed under mild
reaction conditions and it was easy to achieve high oxidation conversions by increasing
reaction temperature or reaction time. The sulfur removal rate of C4H4S
was enhanced when phase transfer catalyst emulsifier OP or tetrabutylammonium bromide
(TBAB) was added. Interestingly, in a H2O2 and formic acid system,
with the addition of TBAB, a bromine substitution trend appeared in the oxidation of C4H4S,
suggesting the influence of TBAB to the oxidation of C4H4S.
Keywords Oxidative desulfurization; thiophene; alumina; phase transfer
catalyst
1. INTRODUCTION
The presence of sulfur compounds in commercial gasoline, for which more than 90% formed
from flow catalytic cracking (FCC) gasoline in China, is highly undesirable since they
result in device corrosion and environmental contamination. Due to the dramatic
environmental impact of sulfur oxides contained in engine exhaust emissions, sulfur
content specifications in both gasoline and diesel pools are becoming more and more
stringent worldwide[1,2]. Faced with continuing fuel quality challenges, the
conventional method of catalytic hydrodesulfurization(HDS) under service conditions for
reducing sulfur content in FCC gasoline is unavoidable. The necessity of producing low
sulfur fuels to meet new regulation mandates will require new desulfurization technique.
Recently, there has been much interest in oxidative desulfurization(ODS) process under low
reaction temperature and pressure.
The ODS process is composed of two stages: oxidation, followed by
liquid extraction. Oxidants can convert sulfur-containing compounds in light oils to much
more polar oxidized species. Such oxidants include nitric acid[3-4], nitrogen
oxides[5], O3[6], H2O2[7-12] et
al. After oxidation, the sulfur compounds are transformed to sulfones. The extraction of
sulfones is considered to be useful method for removal of sulfur compounds[3-4].
The reactivity of sulfur compounds for oxidation is increased with electron density on
sulfur atom. Otsuki et al.[7] have reported the thiophene and thiophene
derivatives with lower electron densities on the sulfur atoms could not be oxidized at 50
°C, while dibenzothiophenes with higher electron densities could be oxidized. This is in
accordance with the conventional thinking that thiophene cannot be oxidized by H2O2
under mild conditions owing to its aromaticity.
Sulfur-containing compounds of FCC gasoline are given in Table 1. The
content of thiophenic sulfur compounds was more than 80% of sulfur-containing compounds
that existed in flow catalytic cracking(FCC) gasoline. Specifically, the chosen sulfur
compounds were C4H4S which could not be oxidized at 50 °C according
to Otsuki[7]. In the present work, the oxidative desulfurization of C4H4S
was studied in H2O2/ formic acid system, particularly, the influence
of the metal-loaded alumina and catalyst to the oxidation of C4H4S.
The research was conducted on simplified model systems of C4H4S
selected from the most representative of those contained in FCC gasoline, dissolved in
different organic solvents.
sulfur-containing compounds |
content |
thiophene |
10.77 |
methylthiophene |
45.93 |
dimethylthiophene |
25.17 |
trimethylthiophene |
4.63 |
tetramethylthiophene |
0.25 |
tetrahydro-thiophene |
3.18 |
mercaptan |
0.79 |
sulfide |
1.18 |
benzothiophene |
7.98 |
2. EXPERIMENTAL
2.1. Materials
Xylene(isomers)and n-heptane were chosen as a representative of the most important
hydrocarbons classes constituting the matrixes of light distillates. The organic solvents
used in this study were formic acid, N,N-dimethylformamide. The phase transfer
catalysts(PTC) used were emulsifier OP, sodium dodecyl benzene sulfonate (SDBS),
tetrabutyl ammonium bromide(TBAB), polyglycol-400.
The sulfur compound selected was C4H4S that was
among those found more frequently in the light distillates from which commercial gasoline
pools are produced. Hydrogen peroxide (30%), alumina, Cu(Ac)2 , Co(Ac)2,
Ni(NO)3, Ce(NO)3 and BaCl2 were supplied by Tianji
Reagent Company. Before use, the concentration of H2O2 was
determined by iodometry. All the products were commercial reagent grade.
2.2. Procedure
C4H4S was dissolved into xylene(isomers) or n-heptane to make a
stock solution with a sulfur content of 500μg/mL. 50
mL the stock solution, 5 mL formic acid and 0.1g metal-loaded alumina were put in a 100 mL
three-necked flask equipped with a magnetic stirrer and reflux condenser. The system was
heated in a thermostatic bath under stirring with a magnetic stirrer at about 1500 rpm.
When the mixture reached the selected reaction temperature (50°C), 5 mL of H2O2
and PTC was then added and the reaction was started. Since the mixture was a heterogeneous
system of three phases (an organic phase, an aqueous phase and solid phase), efficient
mixing was necessary to ensure a homogeneous composition of the bulk liquids.
To determine the initial and residual concentration of C4H4S
in the organic phase, approximately 0.5 mL aliquots of liquid samples were withdrawn from
the reactor at fixed time intervals and after phase separation the organic phase was
analyzed by HP 6890 gas chromatograph (GC) equipped with a flame photometric detector
(FPD) and a flame ionic detector (FID) using a 30m, i.d. 0.32 mm SE-30 column. The main
parameters were the following: carrier gas, nitrogen with a flow of 2 mL/min; oven
temperature, 180 °C; injector temperature, 200 °C; detector temperature, 230 °C; split
ratio, 1/00.
3. RESULTS AND DISCUSSION
3.1. Evaluation of various alumina loaded with metal for oxidation of thiophene
A series of experiments were performed to compare the activity of copper-,
cobalt-, nickel- and cerium-loaded alumina as a catalyst for oxidation of C4H4S.
The mixture of n-heptane solution of sulfur compounds and H2O2/formic
acid became two layers after oxidation: oil layer (top), aqueous layer (bottom). The
sulfur removal rates of C4H4S in oil layer are shown as functions of
reaction time in Fig.1.
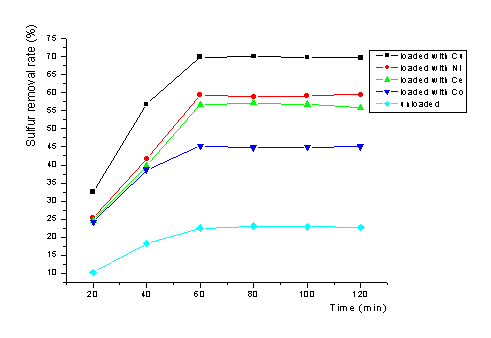
In H2O2/fomic systems, it is clear that metal-loaded alumina is much better compared to alumina. The copper-loaded alumina was very active for the oxidation of C4H4S with 70.1% sulfur removal rate, while the nickel- and cerium-loaded alumina were less active, the sulfur removal rate were 59.3% and 57.1% respectively. The cobalt-loaded alumina was the least active for the oxidation reaction with 45.2% sulfur removal rate. The sulfur removal rate of the oxidized oil layer was the same when N,N-dimethylformamide was used as the extraction solvent. There were no new peaks of the productin GC-FPD analysis in oil layer after oxidation. And deposition occurs obviously in aqueous layer when BaCl2 is added. This phenomenon indicated that the sulfur of C4H4S has been converted to SO42- in the process of oxidation.
3.2 Influence of reaction temperature to the oxidation of thiophene
The oxidation of n-heptane solution of C4H4S was studied in H2O2/fomic systems as the reaction temperature varied from 293K to 393K. The copper-loaded alumina was used as a catalyst in the oxidation. Fig. 2 showed the influence of reaction temperature to oxidation of C4H4S.
The result indicated that lower reaction temperature (293K) was unfit for oxidation of C4H4S. The sulfur removal rate of C4H4S was enhanced with the increase of reaction temperature. The sulfur removal rate of C4H4S reached 70.1% when reaction temperature was 323K. When the reaction temperature exceeded 323K, the conversion of C4H4S fell due to solvent evaporation.
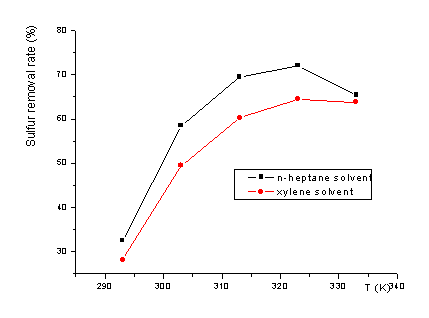
Fig.2 Influence of reaction temperature on oxidation of C4H4S
3.3 Influence of Solvent to the
oxidation of thiophene
Xylene(isomers) and n-heptane were chosen as the organic solvents in the oxidation of C4H4S
in H2O2/fomic systems. The copper-alumina loaded was used as a
catalyst in the oxidation. The oxidation behavior in different solvents was shown in
Fig.3.
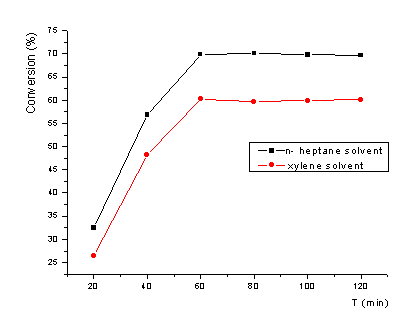
Fig.3 Influence of solvent on the oxidation
of C4H4S
It can be seen from Fig.
3, the sulfur removal rate was lower in solvent xylene than in solvent n-heptane. Low
sulfur removal rate of C4H4S could be resulted by the competition of
solvent xylene and C4H4S on catalyst.
3.4. Influence of phase transfer catalyst to the oxidation of thiophene
Since the reaction system was heterogeneous with three phases, the oxidation reaction
should be improved by PTC. The oxidation of n-heptane solution of C4H4S
was studied over copper-loaded alumina in H2O2/fomic systems when
PTC was added. Table 2 showed the influence of PTC on oxidation of C4H4S.
PTC |
Emulsifier OP |
SDBS |
TBAB |
Polyglycol-400 |
Without PTC |
Sulfur removal rate(%) |
91.3 |
74.8 |
86.5 |
70.2 |
70.1 |
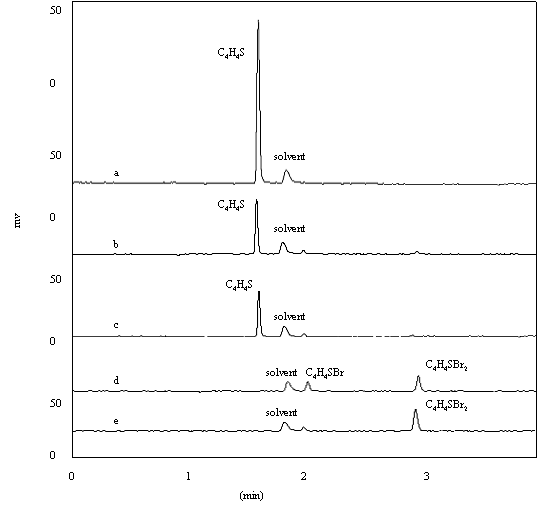
Fig .4 GC-FPD chromatogram of thiophene solution ( a- before oxidation; b- after oxidation (without TBAB); c- after oxidation (0.02gTBAB added); d- after oxidation (0.2gTBAB added); e- after oxidation (0.5gTBAB added) )
Fig.(4c,d,e) indicate that the bromine substitution increases as the concentration of TBAB increases. A part of C4H4S was oxidized, and the other was reacted to form bromine substituted C4H4S when added TBAB was over 0.2g. Sulfur-containing compounds in the oil layer after oxidation was extracted with N,N-dimethylformamide. The sulfur removal rate was 100% in the oil layer after extraction.
4. CONCLUSIONS
(1) C4H4S was oxidized in H2O2 /formic acid
over a series of catalysts of metal-loaded alumina. The copper-loaded alumina was most
active for oxidation of C4H4S in H2O2/ formic
acid system, while the nickel- and cerium-loaded alumina was less active. The
cobalt-loaded alumina was the least active for the oxidation reaction.
(2) The lower reaction temperature (293K) was unfit for oxidation of C4H4S.
The sulfur removal rate of C4H4S was enhanced with the increase of
reaction temperature.
(3) The conversions of C4H4S are lower in solvent xylene than in
solvent n-heptane due to the competition of solvent xylene and thiophene on catalyst.
(4) Emulsifier OP was the most effective PTC with 91.3% sulfur removal rate in the
oxidized oil layer. The bromine substitution of C4H4S occurs when
TBAB added in the H2O2/formic acid system. The sulfur removal rate
of C4H4S was 100% in the oil layer after extraction with
N,N-dimethylformamide.
Acknowledgment Authors are grateful for the financial support from national natural science foundation of china (20276015) and natural science foundation of Hebei Province(203364).
REFERENCES
[1] Avidan, A.; Klein, B.; Ragsdale, R. Hydrocarbon Process. 2001,February, 47-48.
[2] Frederick, C. Hydrocarbon Process. 2002, February, 45-46.
[3] Tam, P.S.; Kittrell, J.R.; Eldridge, J.W. Ind. Eng. Chem. Res.1990,29,321-324.
[4] Tam, P.S.; Kittrell, J.R.; Eldridge, J.W. Ind. Eng. Chem. Res.1990,29,324-329.
[5] Tam, P.S.; Kittrell, J.R.. U. S. Patent 4,485,007,1984.
[6] Paybarah A.; Bone R. L.; Corcoran W. H. Ind. Eng. Chem. Process. Res. Dev.
1982,21,426-428.
[7] Otsuki, S.; Nonaka, T.; Takashima, N.; Qian, W.; Ishihara, A.;Imai, T.; Kabe, T.
Energy Fuels 2000, 14, 1232-1239.
[8] Te, M.; Fairbridge, C.; Ring, Z. Appl. Catal. A: General 2001, 219, 267-280.
[9] Collins, F. M.; Lucy, A. R.; Sharp, C. J. Mol. Catal. A 1997, 117, 397-403.
[10] Mei, H.; Mei, B. W. and Yen, T. F.. Fuel , 2003,82: 405–414.
[11] Shiraishi, Y.; Tachibana, K.; Hirai, T. and Komasawa, I.. Ind. Eng. Chem. Res.,
2002,41, 4362-4375.
[12] Shiraishi, Y. and Hirai, T.. Energy Fuels 2004, 18, 37-40.
负载金属氧化铝和相转移催化剂氧化噻吩的研究
陈兰菊1,2 郭绍辉1 赵地顺2
(1石油大学(北京)重质油加工国家重点实验室,北京 昌平
100022; 2河北科技大学化学系,河北 石家庄 050018)
摘要
以负载金属的氧化铝为催化剂,在H2O2-HCOOH体系中,对催化裂化汽油中特征含硫化合物噻吩的正己烷溶液进行了氧化脱硫研究。考察了负载金属种类、氧化温度、溶剂和相转移催化剂等因素对噻吩脱硫的影响。实验结果表明:在H2O2-HCOOH体系中,负载铜的氧化铝催化活性最好;提高反应温度或延长反应时间可提高噻吩的转化率;相转移催化剂乳化剂OP和四丁基溴化胺(TBAB)的加入可提高噻吩硫的脱除率。值得提出的是,随着TBAB加入量的增多,氧化过程出现了噻吩的溴代产物,这说明TBAB对噻吩氧化的影响随其加入量的增加而增大。
关键词 氧化脱硫;噻吩;氧化铝;相转移催化剂